Posttranslational glutathiolation of aldose reductase (AKR1B1): a possible mechanism of protein recovery from S-nitrosylation
- PMID: 19061876
- PMCID: PMC2929757
- DOI: 10.1016/j.cbi.2008.11.007
Posttranslational glutathiolation of aldose reductase (AKR1B1): a possible mechanism of protein recovery from S-nitrosylation
Abstract
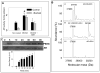
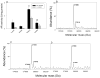
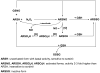
Nitric oxide (NO) is an important regulator of the catalytic activity of aldose reductase (AR). It reacts with the active site cysteines of AR and this reaction results in the formation of several kinetically distinct forms of the protein. The catalytic activity of AR is increased in the ischemic heart and this increase in activity is associated with NO-dependent modification of AR. During reperfusion, the enzyme reverts back to its un-activated form. Although, AR activation has been linked to thiol oxidation, the mechanisms of de-activation remain unclear. Here we report that treatment of recombinant human AR (AKR1B1) by a non-thiol-based NO-donor (DEANO) results in activation and S-nitrosylation of the protein. The nitrosylated (ARSNO), but not the reduced (ARSH), protein reacted with reduced glutathione (GSH) and this reaction resulted in the formation of glutathiolated AR (ARSSG). The modification of AR by NO was site-specific at Cys-298 and was not affected by selective mutation of the neighboring residue, Cys-303 to an alanine. Incubation of the glutathiolated AR (ARSSG) with GSH resulted in the regeneration of the reduced form of the protein (ARSH). Treatment of nitrosylated AR (ARSNO) with ascorbic acid also led to the conversion of the protein to its reduced form. These observations suggest that intracellular reductants such as GSH and ascorbate could convert the nitrosylated form of AR to its basal or reduced state. In general, such reductive reactions might represent a common mechanism for denitrosylating proteins or an "off" switch in NO-mediated signaling pathways involving protein S-nitrosylation reactions.
Figures



References
-
- Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–392. - PubMed
-
- Nakayama M, Nakamura J, Hamada J, Chaya S, Mizubayashi R, Yasuda Y, Kamiya K, Koh N, Hotta N. Aldose reductase inhibition ameliorates pupillary light reflex and F-wave latency in patients with mild diabetic neuropathy. Diabetes Care. 2001;24:1093–1098. - PubMed
-
- Hodgkinson AD, Sondergaard KL, Yang BM, Cross DF, Millward BA, Demaine AG. Aldose reductase expression is induced by hyperglycemia in diabetic nephropathy. Kidney Int. 2001;60:211–218. - PubMed
-
- Chung SSM, Chung SK. Aldose reductase in diabetic microvascular complications. Curr Drug Targets. 2005;6:475–486. - PubMed
-
- Kaiserova K, Srivastava S, Hoetker JD, Awe SO, Tang XL, Cai J, Bhatnagar A. Redox activation of aldose reductase in the ischemic heart. J Biol Chem. 2006;281:15110–15120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

