Sensory regulation of C. elegans male mate-searching behavior
- PMID: 19062284
- PMCID: PMC2652568
- DOI: 10.1016/j.cub.2008.10.050
Sensory regulation of C. elegans male mate-searching behavior
Abstract
How do animals integrate internal drives and external environmental cues to coordinate behaviors? We address this question by studying mate-searching behavior in C. elegans. C. elegans males explore their environment in search of mates (hermaphrodites) and will leave food if mating partners are absent. However, when mates and food coincide, male exploratory behavior is suppressed and males are retained on the food source. We show that the drive to explore is stimulated by male-specific neurons in the tail, the ray neurons. Periodic contact with the hermaphrodite detected through ray neurons changes the male's behavior during periods of no contact and prevents the male from leaving the food source. The hermaphrodite signal is conveyed by male-specific interneurons that are postsynaptic to the rays and that send processes to the major integrative center in the head. This study identifies key parts of the neural circuit that regulates a sexual appetitive behavior in C. elegans.
Figures
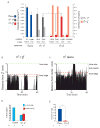
 indicates p< 0.001 when PL values are compared to each-other. (B) Male behaviour with a hermaphrodite. The position is shown of a single male on a food patch during an hour interval. In the presence of hermaphrodites, a male alternates between bouts of contact and bouts on the food edge. The graph plots a male’s distance from one hermaphrodite in the center of the plate (in mm) against time (min). Position was scored every 5 seconds. The food edge and the frame edge are marked. (C) Male behaviour alone. A male alone spends most of its time on the food edge and beyond. Events scored as exits are indicated by the brackets. The graph plots a male’s distance from the center of the plate (in mm) against time (min). The food edge and the frame edge are marked. (D) Males spend a significant amount of time on the food edge when they are in the presence of hermaphrodites but less time than when they are alone. For each condition, four or five males were analysed during observation periods of 1 hour and 30 minutes. (E) Males produce significantly more exit events per minute on the food edge when alone than when in the presence of hermaphrodites. For each condition, four or five males were analysed during observation periods of 1 hour and 30 minutes.
indicates p< 0.001 when PL values are compared to each-other. (B) Male behaviour with a hermaphrodite. The position is shown of a single male on a food patch during an hour interval. In the presence of hermaphrodites, a male alternates between bouts of contact and bouts on the food edge. The graph plots a male’s distance from one hermaphrodite in the center of the plate (in mm) against time (min). Position was scored every 5 seconds. The food edge and the frame edge are marked. (C) Male behaviour alone. A male alone spends most of its time on the food edge and beyond. Events scored as exits are indicated by the brackets. The graph plots a male’s distance from the center of the plate (in mm) against time (min). The food edge and the frame edge are marked. (D) Males spend a significant amount of time on the food edge when they are in the presence of hermaphrodites but less time than when they are alone. For each condition, four or five males were analysed during observation periods of 1 hour and 30 minutes. (E) Males produce significantly more exit events per minute on the food edge when alone than when in the presence of hermaphrodites. For each condition, four or five males were analysed during observation periods of 1 hour and 30 minutes.  indicates p< 0.003 compared to each-other (t-test).
indicates p< 0.003 compared to each-other (t-test).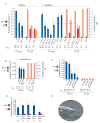
 indicates p< 0.002 when PL values are compared to controls (wt or mock ablated wt and ocr-2 males with hermaphrodites) (B) The spicule neurons are not required for male exploratory behaviour or for retention. PL and Cr values alone and in the presence of hermaphrodites for males with intact, cauterized, or ablated spicule-associated sensory neurons (SPD, SPV and SPC). Ablations were performed in young adults in a syIs33( P(gpa-1)∷gfp) background to visualize the spicule sensory neurons. Mock animals underwent the same manipulations as ablated animals with the exception of not being shot with the laser microbeam. These manipulations slightly reduced the rate of leaving alone compared to non-manipulated males. (C) RnA and RnB neurons stimulate male exploratory behaviour away from food. PL values, alone and in the presence of hermaphrodites, for males with and without RnA or RnB neurons and pkd-2(sy606);lov-1(sy582) double mutants. Only males in which all RnB neurons were dead were assayed. RnA death was not complete. Strains used: bxIs14( P(pkd-2)∷gfp); bxEx136 [P(pkd-2)∷ICE+P(unc-122)∷gfp] for RnB genetic ablations; bxEx137[P(trp-4)∷caspase3-NZ+P(grd-13)∷CZ-caspase3+P(elt-2)∷gfp] or bxEx138[P(trp-4)∷ ICE+P(elt-2)∷gfp] for RnA genetic ablations; and bxEx136;bxEx137 or bxEx136;bxEx138 for RnA,RnB genetic ablations. * indicates p< 0.001 when PL values are compared to wt males alone;
indicates p< 0.002 when PL values are compared to controls (wt or mock ablated wt and ocr-2 males with hermaphrodites) (B) The spicule neurons are not required for male exploratory behaviour or for retention. PL and Cr values alone and in the presence of hermaphrodites for males with intact, cauterized, or ablated spicule-associated sensory neurons (SPD, SPV and SPC). Ablations were performed in young adults in a syIs33( P(gpa-1)∷gfp) background to visualize the spicule sensory neurons. Mock animals underwent the same manipulations as ablated animals with the exception of not being shot with the laser microbeam. These manipulations slightly reduced the rate of leaving alone compared to non-manipulated males. (C) RnA and RnB neurons stimulate male exploratory behaviour away from food. PL values, alone and in the presence of hermaphrodites, for males with and without RnA or RnB neurons and pkd-2(sy606);lov-1(sy582) double mutants. Only males in which all RnB neurons were dead were assayed. RnA death was not complete. Strains used: bxIs14( P(pkd-2)∷gfp); bxEx136 [P(pkd-2)∷ICE+P(unc-122)∷gfp] for RnB genetic ablations; bxEx137[P(trp-4)∷caspase3-NZ+P(grd-13)∷CZ-caspase3+P(elt-2)∷gfp] or bxEx138[P(trp-4)∷ ICE+P(elt-2)∷gfp] for RnA genetic ablations; and bxEx136;bxEx137 or bxEx136;bxEx138 for RnA,RnB genetic ablations. * indicates p< 0.001 when PL values are compared to wt males alone;  indicates p< 0.02 when PL values are compared to each-other. (D) Loss of all RnB neurons and most RnA neurons results in a reduction of exit events at the food edge. Bars plot the average of exit events per minute spent on food edge per worm in 1h 30 min observation periods alone and in the presence of hermaphrodites; * indicates p< 0.05 compared to intact males alone (t-test). (E) DIC photograph of a male tail with the ventral side in focus; posterior is oriented toward the left. All the male specific sensilla are labelled. Dashed lines indicate the position of the spicules, which remain retracted inside the gubernaculum.
indicates p< 0.02 when PL values are compared to each-other. (D) Loss of all RnB neurons and most RnA neurons results in a reduction of exit events at the food edge. Bars plot the average of exit events per minute spent on food edge per worm in 1h 30 min observation periods alone and in the presence of hermaphrodites; * indicates p< 0.05 compared to intact males alone (t-test). (E) DIC photograph of a male tail with the ventral side in focus; posterior is oriented toward the left. All the male specific sensilla are labelled. Dashed lines indicate the position of the spicules, which remain retracted inside the gubernaculum.

 indicates p< 0.001 when PL values are compared to wt hermaphrodites;
indicates p< 0.001 when PL values are compared to wt hermaphrodites;  indicates p< 0.001 when PL values are compared to wt males with hermaphrodites; * * indicates p< 0.03 when PL values are compared to each other.
indicates p< 0.001 when PL values are compared to wt males with hermaphrodites; * * indicates p< 0.03 when PL values are compared to each other.References
-
- White JQ, Nicholas TJ, Gritton J, Truong L, Davidson ER, Jorgensen EM. The Sensory Circuitry for Sexual Attraction in C. elegans Males. Curr Biol 2007 - PubMed
-
- Maloof JN, Kenyon C. The Hox gene lin-39 is required during C. elegans vulval induction to select the outcome of Ras signaling. Development. 1998;125:181–190. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

