The homotetrameric kinesin-5 KLP61F preferentially crosslinks microtubules into antiparallel orientations
- PMID: 19062285
- PMCID: PMC2657206
- DOI: 10.1016/j.cub.2008.10.026
The homotetrameric kinesin-5 KLP61F preferentially crosslinks microtubules into antiparallel orientations
Abstract
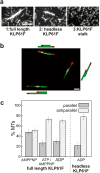
The segregation of genetic material during mitosis is coordinated by the mitotic spindle, whose action depends upon the polarity patterns of its microtubules (MTs). Homotetrameric mitotic kinesin-5 motors can crosslink and slide adjacent spindle MTs, but it is unknown whether they or other motors contribute to establishing these MT polarity patterns. Here, we explored whether the Drosophila embryo kinesin-5 KLP61F, which plausibly crosslinks both parallel and antiparallel MTs, displays a preference for parallel or antiparallel MT orientation. In motility assays, KLP61F was observed to crosslink and slide adjacent MTs, as predicted. Remarkably, KLP61F displayed a 3-fold higher preference for crosslinking MTs in the antiparallel orientation. This polarity preference was observed in the presence of ADP or ATP plus AMPPNP, but not AMPPNP alone, which induces instantaneous rigor binding. Also, a purified motorless tetramer containing the C-terminal tail domains displayed an antiparallel orientation preference, confirming that motor activity is not required. The results suggest that, during morphogenesis of the Drosophila embryo mitotic spindle, KLP61F's crosslinking and sliding activities could facilitate the gradual accumulation of KLP61F within antiparallel interpolar MTs at the equator, where the motor could generate force to drive poleward flux and pole-pole separation.
Figures

References
-
- Brust-Mascher I, Scholey JM. Mitotic spindle dynamics in Drosophila. Int Rev Cytol. 2007;259:139–172. - PubMed
-
- Walczak CE, Heald R. Mechanisms of mitotic spindle assembly and function. Int Rev Cytol. 2008;265:111–158. - PubMed
-
- Kapitein LC, Peterman EJ, Kwok BH, Kim JH, Kapoor TM, Schmidt CF. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature. 2005;435:114–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

