Morphogenetic and regulatory mechanisms during developmental chondrogenesis: new paradigms for cartilage tissue engineering
- PMID: 19063663
- PMCID: PMC2817664
- DOI: 10.1089/ten.teb.2008.0329
Morphogenetic and regulatory mechanisms during developmental chondrogenesis: new paradigms for cartilage tissue engineering
Abstract
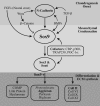
Cartilage is the first skeletal tissue to be formed during embryogenesis leading to the creation of all mature cartilages and bones, with the exception of the flat bones in the skull. Therefore, errors occurring during the process of chondrogenesis, the formation of cartilage, often lead to severe skeletal malformations such as dysplasias. There are hundreds of skeletal dysplasias, and the molecular genetic etiology of some remains more elusive than of others. Many efforts have aimed at understanding the morphogenetic event of chondrogenesis in normal individuals, of which the main morphogenetic and regulatory mechanisms will be reviewed here. For instance, many signaling molecules that guide chondrogenesis--for example, transforming growth factor-beta, bone morphogenetic proteins, fibroblast growth factors, and Wnts, as well as transcriptional regulators such as the Sox family--have already been identified. Moreover, extracellular matrix components also play an important role in this developmental event, as evidenced by the promotion of the chondrogenic potential of chondroprogenitor cells caused by collagen II and proteoglycans like versican. The growing evidence of the elements that control chondrogenesis and the increasing number of different sources of progenitor cells will, hopefully, help to create tissue engineering platforms that could overcome many developmental or degenerative diseases associated with cartilage defects.
Figures
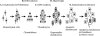


References
-
- Newman S.A. Frisch H.L. Dynamics of skeletal pattern formation in developing chick limb. Science. 1979;205:662. - PubMed
-
- Horton W.A. Hood O.J. Machado M.A. Ahmed S. Griffey E.S. Abnormal ossification in thanatophoric dysplasia. Bone. 1988;9:53. - PubMed
-
- Hall B.K. Miyake T. Divide, accumulate, differentiate: cell condensation in skeletal development revisited. Int J Dev Biol. 1995;39:881. - PubMed
-
- Hoffman L.M. Weston A.D. Underhill T.M. Molecular mechanisms regulating chondroblast differentiation. J Bone Joint Surg Am. 2003;85-A Suppl 2:124. - PubMed
-
- Mackie E.J. Ahmed Y.A. Tatarczuch L. Chen K.S. Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40:46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

