muCT-based measurement of cortical bone graft-to-host union
- PMID: 19063685
- PMCID: PMC2672207
- DOI: 10.1359/jbmr.081232
muCT-based measurement of cortical bone graft-to-host union
Abstract
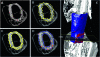
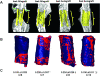
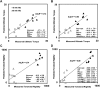
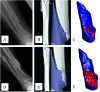
Evaluation of structural bone grafts risk of failure requires noninvasive quantitative predictors of functional strength. We hypothesized that a quantitative graft-to-host union biometric would correlate significantly with biomechanical properties as a surrogate for the risk of fracture. To test this, we developed a novel algorithm to compute the union between host callus and graft, which was termed the union ratio. We compared the union ratio of live autografts to devitalized allografts implanted into the mid-diaphysis of mouse femurs for 6 and 9 wk. Surprisingly, the autograft union ratio decreased from 0.228 +/- 0.029 at 6 wk to 0.15 +/- 0.011 at 9 wk (p < 0.05) and did not correlate with the torsional properties of the autografts. The allograft union ratio was 0.105 +/- 0.023 at 6 wk but increased to 0.224 +/- 0.029 at 9 wk (p < 0.05). As a single variable, the union ratio correlated significantly with ultimate torque (R (2) = 0.58) and torsional rigidity (R (2) = 0.51) of the allografts. Multivariable regression analyses of allografts that included the union ratio, the graft bone volume, the maximum and minimum polar moment of inertia, and their first-order interaction terms with the union ratio as independent variables resulted in significant correlations with the ultimate torque and torsional rigidity (adjusted R (2) = 0.80 and 0.89, respectively). These results suggest that, unlike live autografts, the union between the devitalized allograft and host contributes significantly to the strength of grafted bone. The union ratio has important clinical implications as a novel biometric for noninvasive assessment of functional strength and failure risk.
Figures


References
-
- Brigman BE, Hornicek FJ, Gebhardt MC, Mankin HJ. Allografts about the knee in young patients with high-grade sarcoma. Clin Orthop Relat Res. 2004:232–239. - PubMed
-
- Mohler DG, Yaszay B, Hong R, Wera G. Intercalary tibial allografts following tumor resection: The role of fibular centralization. Orthopedics. 2003;26:631–637. - PubMed
-
- Hornicek FJ, Gebhardt MC, Tomford WW, Sorger JI, Zavatta M, Menzner JP, Mankin HJ. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001:87–98. - PubMed
-
- Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005:36–42. - PubMed
-
- Tiyapatanaputi P, Rubery PT, Carmouche J, Schwarz EM, O'Keefe RJ, Zhang X. A novel murine segmental femoral graft model. J Orthop Res. 2004;22:1254–1260. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

