Inflammation and NFkappaB activation is decreased by hypothermia following global cerebral ischemia
- PMID: 19063968
- PMCID: PMC2737398
- DOI: 10.1016/j.nbd.2008.11.001
Inflammation and NFkappaB activation is decreased by hypothermia following global cerebral ischemia
Abstract
We previously showed that hypothermia attenuates inflammation in focal cerebral ischemia (FCI) by suppressing activating kinases of nuclear factor-kappa B (NFkappaB). Here we characterize the inflammatory response in global cerebral ischemia (GCI), and the influence of mild hypothermia. Rodents were subjected to GCI by bilateral carotid artery occlusion. The inflammatory response was accompanied by microglial activation, but not neutrophil infiltration, or blood brain barrier disruption. Mild hypothermia reduced CA1 damage, decreased microglial activation and decreased nuclear NFkappaB translocation and activation. Similar anti-inflammatory effects of hypothermia were observed in a model of pure brain inflammation that does not cause brain cell death. Primary microglial cultures subjected to oxygen glucose deprivation (OGD) or stimulated with LPS under hypothermic conditions also experienced less activation and less NFkappaB translocation. However, NFkappaB regulatory proteins were not affected by hypothermia. The inflammatory response following GCI and hypothermia's anti-inflammatory mechanism is different from that observed in FCI.
Figures






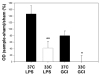


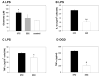
References
-
- Aloia RC, Raison JK. Membrane function in mammalian hibernation. Biochim Biophys Acta. 1989;988:123–146. - PubMed
-
- Baker CJ, Onesti ST, Solomon RA. Reduction by delayed hypothermia of cerebral infarction following middle cerebral artery occlusion in the rat: a time-course study. J Neurosurg. 1992;77:438–444. - PubMed
-
- Beck J, Stummer W, Lehmberg J, Baethmann A, Uhl E. Activation of leukocyte-endothelial interactions and reduction of selective neuronal death after global cerebral ischemia. Neurosci Lett. 2007;414:159–164. - PubMed
-
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. - PubMed
-
- Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke. 1989;20:904–910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

