Neuronal Ca2+ sensor VILIP-1 leads to the upregulation of functional alpha4beta2 nicotinic acetylcholine receptors in hippocampal neurons
- PMID: 19063970
- PMCID: PMC2683982
- DOI: 10.1016/j.mcn.2008.11.001
Neuronal Ca2+ sensor VILIP-1 leads to the upregulation of functional alpha4beta2 nicotinic acetylcholine receptors in hippocampal neurons
Abstract
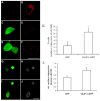
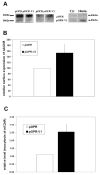
The neuronal Ca2+-sensor protein VILIP-1, known to affect clathrin-dependent receptor trafficking, has been shown to interact with the cytoplasmic loop of the alpha4-subunit of the alpha4beta2 nicotinic acetylcholine receptor (nAChR), which is the most abundant nAChR subtype with high-affinity for nicotine in the brain. The alpha4beta2 nAChR is crucial for nicotine addiction and the beneficial effects of nicotine on cognition. Its dysfunction has been implicated in frontal lobe epilepsy, Alzheimer's disease and schizophrenia. Here we report that overexpression of VILIP-1 enhances ACh responsiveness, whereas siRNA against VILIP-1 reduces alpha4beta2 nAChR currents of hippocampal neurons. The underlying molecular mechanism likely involves enhanced constitutive exocytosis of alpha4beta2 nAChRs mediated by VILIP-1. The two interaction partners co-localize in a Ca2+-dependent manner with syntaxin-6, a Golgi-SNARE protein involved in trans-Golgi membrane trafficking. Thus, we speculate that regulation of VILIP-1-expression might modulate surface expression of ligand-gated ion channels, such as the alpha4beta2 nAChRs, possibly comprising a novel form of physiological up-regulation of ligand-gated ion channels.
Figures





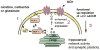
References
-
- Ames JB, Ishima R, Tanaka T, Gordon JI, Stryer L, Ikura M. Molecular mechanics of calcium-myristoyl switches. Nature. 1997;389:198–202. - PubMed
-
- Bernstein HG, Becker A, Keilhoff G, Gorcyca WA, Braunewell KH, Grecksch G. Ketamine induced changes of the expression of neuronal calcium sensor proteins VILIP-1, -3 and hippocalcin in a partial schizophrenia model in rats. Neurosci Lett. 2003;339:95–98. - PubMed
-
- Bernstein HG, Braunewell KH, Spilker C, Danos P, Baumann B, Diekmann S, Gundelfinger ED, Bogerts B. Hippocampal expression of the Ca2+ sensor protein VILIP-1 in schizophrenia. Neuroreport. 2002;23:393–396. - PubMed
-
- Blondeau F, Ritter B, Allaire PD, Wasiak S, Girard M, Hussain NK, Angers A, Legendre-Guillemin V, Roy L, Boismenu D, Kearney RE, Bell AW, Bergeron JJ, McPherson PS. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc Natl Acad Sci U S A. 2004;101:3833–3838. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

