Zebrafish and medaka: model organisms for a comparative developmental approach of brain asymmetry
- PMID: 19064351
- PMCID: PMC2666085
- DOI: 10.1098/rstb.2008.0260
Zebrafish and medaka: model organisms for a comparative developmental approach of brain asymmetry
Abstract
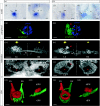
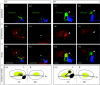
Comparison between related species is a successful approach to uncover conserved and divergent principles of development. Here, we studied the pattern of epithalamic asymmetry in zebrafish (Danio rerio) and medaka (Oryzias latipes), two related teleost species with 115-200 Myr of independent evolution. We found that these species share a strikingly conserved overall pattern of asymmetry in the parapineal-habenular-interpeduncular system. Nodal signalling exhibits comparable spatial and temporal asymmetric expressions in the presumptive epithalamus preceding the development of morphological asymmetries. Neuroanatomical asymmetries consist of left-sided asymmetric positioning and connectivity of the parapineal organ, enlargement of neuropil in the left habenula compared with the right habenula and segregation of left-right habenular efferents along the dorsoventral axis of the interpeduncular nucleus. Despite the overall conservation of asymmetry, we observed heterotopic changes in the topology of parapineal efferent connectivity, heterochronic shifts in the timing of developmental events underlying the establishment of asymmetry and divergent degrees of canalization of embryo laterality. We offer new tools for developmental time comparison among species and propose, for each of these transformations, novel hypotheses of ontogenic mechanisms that explain interspecies variations that can be tested experimentally. Together, these findings highlight the usefulness of zebrafish and medaka as comparative models to study the developmental mechanisms of epithalamic asymmetry in vertebrates.
Figures


References
-
- Aizawa H., Bianco I.H., Hamaoka T., Miyashita T., Uemura O., Concha M.L., Russell C., Wilson S.W., Okamoto H. Laterotopic representation of left–right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Curr. Biol. 2005;15:238–243. doi:10.1016/j.cub.2005.01.014 - DOI - PMC - PubMed
-
- Aizawa H., Goto M., Sato T., Okamoto H. Temporally regulated asymmetric neurogenesis causes left–right difference in the zebrafish habenular structures. Dev. Cell. 2007;12:87–98. doi:10.1016/j.devcel.2006.10.004 - DOI - PubMed
-
- Barth K.A., Miklosi A., Watkins J., Bianco I.H., Wilson S.W., Andrew R.J. fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr. Biol. 2005;15:844–850. doi:10.1016/j.cub.2005.03.047 - DOI - PMC - PubMed
-
- Bianco I.H., Wilson S.W. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Phil. Trans. R. Soc. B. 2009;364:1005–1020. doi:10.1098/rstb.2008.0213 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

