Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age
- PMID: 19064627
- PMCID: PMC2655100
- DOI: 10.1056/NEJMoa0807381
Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age
Abstract
Background: Plasmodium falciparum malaria is a pressing global health problem. A previous study of the malaria vaccine RTS,S (which targets the circumsporozoite protein), given with an adjuvant system (AS02A), showed a 30% rate of protection against clinical malaria in children 1 to 4 years of age. We evaluated the efficacy of RTS,S given with a more immunogenic adjuvant system (AS01E) in children 5 to 17 months of age, a target population for vaccine licensure.
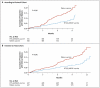
Methods: We conducted a double-blind, randomized trial of RTS,S/AS01E vaccine as compared with rabies vaccine in children in Kilifi, Kenya, and Korogwe, Tanzania. The primary end point was fever with a falciparum parasitemia density of more than 2500 parasites per microliter, and the mean duration of follow-up was 7.9 months (range, 4.5 to 10.5).
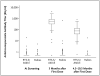
Results: A total of 894 children were randomly assigned to receive the RTS,S/AS01E vaccine or the control (rabies) vaccine. Among the 809 children who completed the study procedures according to the protocol, the cumulative number in whom clinical malaria developed was 32 of 402 assigned to receive RTS,S/AS01E and 66 of 407 assigned to receive the rabies vaccine; the adjusted efficacy rate for RTS,S/AS01E was 53% (95% confidence interval [CI], 28 to 69; P<0.001) on the basis of Cox regression. Overall, there were 38 episodes of clinical malaria among recipients of RTS,S/AS01E, as compared with 86 episodes among recipients of the rabies vaccine, with an adjusted rate of efficacy against all malarial episodes of 56% (95% CI, 31 to 72; P<0.001). All 894 children were included in the intention-to-treat analysis, which showed an unadjusted efficacy rate of 49% (95% CI, 26 to 65; P<0.001). There were fewer serious adverse events among recipients of RTS,S/AS01E, and this reduction was not only due to a difference in the number of admissions directly attributable to malaria.
Conclusions: RTS,S/AS01E shows promise as a candidate malaria vaccine. (ClinicalTrials.gov number, NCT00380393.)
2008 Massachusetts Medical Society
Figures
Comment in
-
A hopeful beginning for malaria vaccines.N Engl J Med. 2008 Dec 11;359(24):2599-601. doi: 10.1056/NEJMe0808983. Epub 2008 Dec 8. N Engl J Med. 2008. PMID: 19064626 No abstract available.
-
Malaria vaccine: the latest news from RTS,S/AS01E vaccine.Expert Rev Vaccines. 2009 Mar;8(3):285-8. doi: 10.1586/14760584.8.3.285. Expert Rev Vaccines. 2009. PMID: 19249969 No abstract available.
-
RTS,S/AS01E vaccine against malaria.N Engl J Med. 2009 Mar 19;360(12):1253; author reply 1253-4. doi: 10.1056/NEJMc090025. N Engl J Med. 2009. PMID: 19297579 No abstract available.
-
Poor control vaccines in two randomised trials of malaria vaccine?Vaccine. 2009 May 14;27(22):2914-5. doi: 10.1016/j.vaccine.2009.03.004. Epub 2009 Mar 13. Vaccine. 2009. PMID: 19366572 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
