The transforming Rho family GTPase Wrch-1 disrupts epithelial cell tight junctions and epithelial morphogenesis
- PMID: 19064640
- PMCID: PMC2643799
- DOI: 10.1128/MCB.00336-08
The transforming Rho family GTPase Wrch-1 disrupts epithelial cell tight junctions and epithelial morphogenesis
Abstract
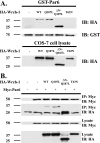
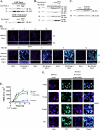
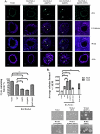
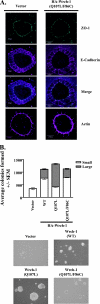
Wrch-1, an atypical and transforming Rho GTPase, regulates cellular activities including proliferation and actin organization, but its functions and effectors remain poorly characterized. We show here that Wrch-1 distributes along the apical and basolateral membranes in MDCK cells and binds the cell polarity protein Par6 in a GTP-dependent manner. Activated Wrch-1 negatively regulates the kinetics of tight junction (TJ) assembly during epithelial cell polarization but has no detectable effect on overall cell polarity in confluent monolayers. It also causes a dramatic cytoskeletal reorganization and multilayering in cells grown in two-dimensional culture and disrupts cystogenesis of cells grown in three-dimensional (3D) culture. Similarly, short hairpin RNA-mediated knockdown of Wrch-1 perturbs cystogenesis in 3D culture, suggesting that tight regulation of Wrch-1 activity is necessary for normal epithelial morphogenesis. A weakly transforming effector domain mutant of activated Wrch-1 that inhibits Par6 binding abrogates the ability of Wrch-1 to disrupt TJ formation, actin organization, and epithelial morphogenesis. We hypothesize that Wrch-1-induced morphological and growth transformation may occur in part through Par6-mediated disruption of TJs and actin organization.
Figures





References
-
- Ando-Akatsuka, Y., S. Yonemura, M. Itoh, M. Furuse, and S. Tsukita. 1999. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J. Cell. Physiol. 179115-125. - PubMed
-
- Aranda, V., T. Haire, M. E. Nolan, J. P. Calarco, A. Z. Rosenberg, J. P. Fawcett, T. Pawson, and S. K. Muthuswamy. 2006. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat. Cell Biol. 81235-1245. - PubMed
-
- Aronheim, A., Y. C. Broder, A. Cohen, A. Fritsch, B. Belisle, and A. Abo. 1998. Chp, a homologue of the GTPase Cdc42Hs, activates the JNK pathway and is implicated in reorganizing the actin cytoskeleton. Curr. Biol. 81125-1128. - PubMed
-
- Berzat, A. C., J. E. Buss, E. J. Chenette, C. A. Weinbaum, A. Shutes, C. J. Der, A. Minden, and A. D. Cox. 2005. Transforming activity of the Rho family GTPase, Wrch-1, a Wnt-regulated Cdc42 homolog, is dependent on a novel carboxyl-terminal palmitoylation motif. J. Biol. Chem. 28033055-33065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
