Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand
- PMID: 19064696
- PMCID: PMC2605231
- DOI: 10.1084/jem.20080201
Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand
Abstract
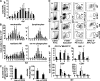
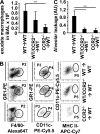
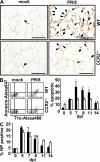
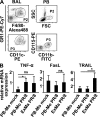
Mononuclear phagocytes have been attributed a crucial role in the host defense toward influenza virus (IV), but their contribution to influenza-induced lung failure is incompletely understood. We demonstrate for the first time that lung-recruited "exudate" macrophages significantly contribute to alveolar epithelial cell (AEC) apoptosis by the release of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in a murine model of influenza-induced pneumonia. Using CC-chemokine receptor 2-deficient (CCR2(-/-)) mice characterized by defective inflammatory macrophage recruitment, and blocking anti-CCR2 antibodies, we show that exudate macrophage accumulation in the lungs of influenza-infected mice is associated with pronounced AEC apoptosis and increased lung leakage and mortality. Among several proapoptotic mediators analyzed, TRAIL messenger RNA was found to be markedly up-regulated in alveolar exudate macrophages as compared with peripheral blood monocytes. Moreover, among the different alveolar-recruited leukocyte subsets, TRAIL protein was predominantly expressed on macrophages. Finally, abrogation of TRAIL signaling in exudate macrophages resulted in significantly reduced AEC apoptosis, attenuated lung leakage, and increased survival upon IV infection. Collectively, these findings demonstrate a key role for exudate macrophages in the induction of alveolar leakage and mortality in IV pneumonia. Epithelial cell apoptosis induced by TRAIL-expressing macrophages is identified as a major underlying mechanism.
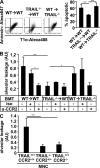
Figures




References
-
- von Wulffen, W., M. Steinmueller, S. Herold, L.M. Marsh, P. Bulau, W. Seeger, T. Welte, J. Lohmeyer, and U.A. Maus. 2007. Lung dendritic cells elicited by Fms-like tyrosine 3-kinase ligand amplify the inflammatory response to lipopolysaccharide. Am. J. Respir. Crit. Care Med. 176:892–901. - PubMed
-
- Fogg, D.K., C. Sibon, C. Miled, S. Jung, P. Aucouturier, D.R. Littman, A. Cumano, and F. Geissmann. 2006. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 311:83–87. - PubMed
-
- Herold, S., W. von Wulffen, M. Steinmueller, S. Pleschka, W.A. Kuziel, M. Mack, M. Srivastava, W. Seeger, U.A. Maus, and J. Lohmeyer. 2006. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J. Immunol. 177:1817–1824. - PubMed
-
- Maus, U., K. von Grote, W.A. Kuziel, M. Mack, E.J. Miller, J. Cihak, M. Stangassinger, R. Maus, D. Schlondorff, W. Seeger, and J. Lohmeyer. 2002. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am. J. Respir. Crit. Care Med. 166:268–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

