An essential role for the MAL protein in targeting Lck to the plasma membrane of human T lymphocytes
- PMID: 19064697
- PMCID: PMC2605221
- DOI: 10.1084/jem.20080552
An essential role for the MAL protein in targeting Lck to the plasma membrane of human T lymphocytes
Abstract
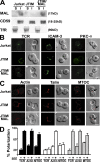
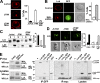
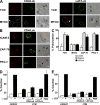
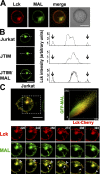
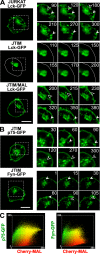
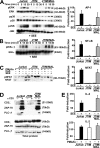
The MAL protein is an essential component of the specialized machinery for apical targeting in epithelial cells. The src family kinase Lck plays a pivotal role in T cell signaling. We show that MAL is required in T cells for efficient expression of Lck at the plasma membrane and activation of IL-2 transcription. To investigate the mechanism by which MAL regulates Lck targeting, we analyzed the dynamics of Lck and found that it travels to the plasma membrane in specific transport carriers containing MAL. Coimmunoprecipitation experiments indicated an association of MAL with Lck. Both carrier formation and partitioning of Lck into detergent-insoluble membranes were ablated in the absence of MAL. Polarization of T cell receptor for antigen (TCR) and microtubule-organizing center to immunological synapse (IS) were also defective. Although partial correction of the latter defects was possible by forced expression of Lck at the plasma membrane, their complete correction, formation of transport vesicles, partitioning of Lck, and restoration of signaling pathways, which are required for IL-2 transcription up-regulation, were achieved by exogenous expression of MAL. We concluded that MAL is required for recruitment of Lck to specialized membranes and formation of specific transport carriers for Lck targeting. This novel transport pathway is crucial for TCR-mediated signaling and IS assembly.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

