Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE
- PMID: 19064698
- PMCID: PMC2605236
- DOI: 10.1084/jem.20081165
Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE
Abstract
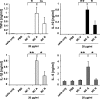
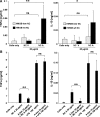
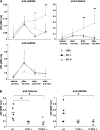
Autoantibodies against double-stranded DNA (dsDNA) and nucleosomes represent a hallmark of systemic lupus erythematosus (SLE). However, the mechanisms involved in breaking the immunological tolerance against these poorly immunogenic nuclear components are not fully understood. Impaired phagocytosis of apoptotic cells with consecutive release of nuclear antigens may contribute to the immune pathogenesis. The architectural chromosomal protein and proinflammatory mediator high mobility group box protein 1 (HMGB1) is tightly attached to the chromatin of apoptotic cells. We demonstrate that HMGB1 remains bound to nucleosomes released from late apoptotic cells in vitro. HMGB1-nucleosome complexes were also detected in plasma from SLE patients. HMGB1-containing nucleosomes from apoptotic cells induced secretion of interleukin (IL) 1beta, IL-6, IL-10, and tumor necrosis factor (TNF) alpha and expression of costimulatory molecules in macrophages and dendritic cells (DC), respectively. Neither HMGB1-free nucleosomes from viable cells nor nucleosomes from apoptotic cells lacking HMGB1 induced cytokine production or DC activation. HMGB1-containing nucleosomes from apoptotic cells induced anti-dsDNA and antihistone IgG responses in a Toll-like receptor (TLR) 2-dependent manner, whereas nucleosomes from living cells did not. In conclusion, HMGB1-nucleosome complexes activate antigen presenting cells and, thereby, may crucially contribute to the pathogenesis of SLE via breaking the immunological tolerance against nucleosomes/dsDNA.
Figures





References
-
- Hahn, B.H. 1998. Antibodies to DNA. N. Engl. J. Med. 338:1359–1368. - PubMed
-
- Ehrenstein, M.R., D.R. Katz, M.H. Griffiths, L. Papadaki, T.H. Winkler, J.R. Kalden, and D.A. Isenberg. 1995. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 48:705–711. - PubMed
-
- Thomas, J.O., and A.A. Travers. 2001. HMG1 and 2, and related ‘architectural’ DNA-binding proteins. Trends Biochem. Sci. 26:167–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

