The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD)
- PMID: 19065671
- PMCID: PMC3063896
- DOI: 10.1002/hep.22636
The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD)
Abstract
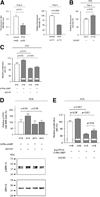
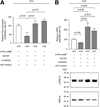
PCK rats, an animal model of autosomal recessive polycystic kidney disease (ARPKD), develop cholangiocyte-derived liver cysts associated with increased intracellular adenosine 3',5'-cyclic adenosine monophosphate (cAMP), the inhibition of which suppresses cyst growth. We hypothesized that elevated cAMP stimulates cholangiocyte proliferation via two downstream effectors, exchange proteins activated by cAMP (Epac1 and Epac2 isoforms) and protein kinase A (PKA), and that intracellular calcium is also involved in this process. Assessment of Epac isoforms and PKA regulatory subunits at the messenger RNA and protein level showed that cultured normal rat cholangiocytes express Epac1, Epac2, and all regulatory PKA subunits. Epac isoforms and the PKA RIbeta subunit were overexpressed in cultured PCK cholangiocytes. Proliferation analysis in response to Epac and PKA activation indicated that both normal and PCK cholangiocytes increase their growth upon Epac-specific stimulation, while PKA-specific stimulation results in differential effects, suppressing proliferation in normal cholangiocytes but accelerating this process in PCK cholangiocytes. On the other hand, both PKA and Epac activation of cystic structures generated by normal and PCK cholangiocytes when cultured under three-dimensional conditions resulted in increased cyst growth, particularly in PCK-cholangiocyte derived cysts. Pharmacological inhibitors and small interfering RNA-mediated gene silencing demonstrated the specificity of each effector activation, as well as the involvement of MEK-ERK1/2 signaling in all the observed effector-associated proliferation changes. Hyperproliferation of PCK cholangiocytes in response to PKA stimulation, but not to Epac stimulation, was found to be associated with decreased intracellular calcium, and restoration of calcium levels blocked the PKA-dependent proliferation via the PI3K/AKT pathway.
Conclusion: Our data provide strong evidence that both cAMP effectors, Epac and PKA, and the levels of intracellular calcium are involved in the hepatic cystogenesis of ARPKD.
Conflict of interest statement
No conflicts of interest exist.
Figures








References
-
- Shneider BL, Magid MS. Liver disease in autosomal recessive polycystic kidney disease. Pediatr Transplant. 2005;9:634–639. - PubMed
-
- Nagasawa Y, Matthiesen S, Onuchic LF, Hou X, Bergmann C, Esquivel E, et al. Identification and characterization of Pkhd1, the mouse orthologue of the human ARPKD gene. J Am Soc Nephrol. 2002;13:2246–2258. - PubMed
-
- Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–269. - PubMed
-
- Masyuk TV, Huang BQ, Ward CJ, Masyuk AI, Yuan D, Splinter PL, et al. Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology. 2003;125:1303–1310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
