Modular synthesis of functional nanoscale coordination polymers
- PMID: 19065692
- PMCID: PMC4040293
- DOI: 10.1002/anie.200803387
Modular synthesis of functional nanoscale coordination polymers
Abstract
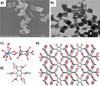
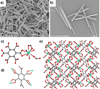
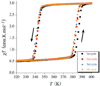
The coordination-directed assembly of metal ions and organic bridging ligands has afforded a variety of bulk-scale hybrid materials with promising characteristics for a number of practical applications, such as gas storage and heterogeneous catalysis. Recently, so-called coordination polymers have emerged as a new class of hybrid nanomaterials. Herein, we highlight advances in the syntheses of both amorphous and crystalline nanoscale coordination polymers. We also illustrate how scaling down these materials to the nano-regime has enabled their use in a broad range of applications including catalysis, spin-crossover, templating, biosensing, biomedical imaging, and anticancer drug delivery. These results underscore the exciting opportunities of developing next-generation functional nanomaterials based on molecular components.
Figures












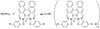

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

