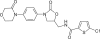New compounds in the management of venous thromboembolism after orthopedic surgery: focus on rivaroxaban
- PMID: 19066002
- PMCID: PMC2597756
- DOI: 10.2147/vhrm.s3550
New compounds in the management of venous thromboembolism after orthopedic surgery: focus on rivaroxaban
Abstract
Rivaroxaban (Xarelto) is a member of a new class of oral, direct (antithrombin-independent) factor Xa inhibitors, which restrict thrombin generation both in vitro and in vivo. After oral administration the absorption is near 100%, the bioavailability is near 80%, and the elimination half-life is 5-9 hours with mixed excretion via the renal and fecal/biliary routes. The pharmacokinetics of rivaroxaban are predictable and consistent with a rapid onset of antithrombotic action within 2 hours after administration. Phase II clinical studies have been carried out in patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA) and a dose of 10 mg once daily for thromboprophylaxis was selected for further clinical development. The results of the phase III studies showed a significantly better antithrombotic efficacy of rivaroxaban compared with enoxaparin both in the short term (10-14 days) in TKA patients and long term (35 +/- 4 days) in THA patients with a comparable safety. Symptomatic thromboembolic events were also significantly reduced with rivaroxaban. Liver enzyme elevation was seen in patients treated with rivaroxaban, but there was no indication of an increased risk of liver toxicity compared with enoxaparin. In conclusion, rivaroxaban is a potent and safe new compound for antithrombotic prophylaxis in orthopedic surgery.
Figures
References
-
- Bergqvist D, Johnsson B. Cost-effectiveness of prolonged administration of a low molecular weight heparin for the prevention of deep venous thrombosis following hip replacement. Value Health. 1999;2:288–94. - PubMed
-
- Dahl OE, Aspelin T, Lyberg T. The role of bone traumatization in the initiation of proximal deep vein thrombosis during cemented hip replacement surgery in pigs. Blood Coagul Fibrinolysis. 1995;6:709–17. - PubMed
-
- Eriksson BI, Dahl OE, Büller HR, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005;3:103–11. - PubMed
-
- Eriksson BI, Borris L, Dahl OE, et al. Oral, direct Factor Xa inhibition with BAY 59–7939 for the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost. 2006a;4:121–8. - PubMed
-
- Eriksson BI, Borris LC, Dahl OE, et al. A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59–7939), for thromboprophylaxis after total hip replacement. Circulation. 2006b;114:2374–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources