Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism
- PMID: 19066218
- PMCID: PMC2598730
- DOI: 10.1073/pnas.0810339105
Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism
Abstract
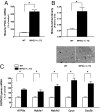
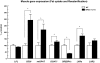
Peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1alpha has been shown to play critical roles in regulating mitochondria biogenesis, respiration, and muscle oxidative phenotype. Furthermore, reductions in the expression of PGC-1alpha in muscle have been implicated in the pathogenesis of type 2 diabetes. To determine the effect of increased muscle-specific PGC-1alpha expression on muscle mitochondrial function and glucose and lipid metabolism in vivo, we examined body composition, energy balance, and liver and muscle insulin sensitivity by hyperinsulinemic-euglycemic clamp studies and muscle energetics by using (31)P magnetic resonance spectroscopy in transgenic mice. Increased expression of PGC-1alpha in muscle resulted in a 2.4-fold increase in mitochondrial density, which was associated with an approximately 60% increase in the unidirectional rate of ATP synthesis. Surprisingly, there was no effect of increased muscle PGC-1alpha expression on whole-body energy expenditure, and PGC-1alpha transgenic mice were more prone to fat-induced insulin resistance because of decreased insulin-stimulated muscle glucose uptake. The reduced insulin-stimulated muscle glucose uptake could most likely be attributed to a relative increase in fatty acid delivery/triglyceride reesterfication, as reflected by increased expression of CD36, acyl-CoA:diacylglycerol acyltransferase1, and mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase, that may have exceeded mitochondrial fatty acid oxidation, resulting in increased intracellular lipid accumulation and an increase in the membrane to cytosol diacylglycerol content. This, in turn, caused activation of PKC, decreased insulin signaling at the level of insulin receptor substrate-1 (IRS-1) tyrosine phosphorylation, and skeletal muscle insulin resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


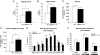
References
-
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. - PubMed
-
- Lillioja S, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: Prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. - PubMed
-
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM: A balanced overview. Diabetes Care. 1992;15:318–368. - PubMed
-
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and β-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

