Targeting hypoxia-inducible factor-1alpha with Tf-PEI-shRNA complex via transferrin receptor-mediated endocytosis inhibits melanoma growth
- PMID: 19066596
- PMCID: PMC2835063
- DOI: 10.1038/mt.2008.266
Targeting hypoxia-inducible factor-1alpha with Tf-PEI-shRNA complex via transferrin receptor-mediated endocytosis inhibits melanoma growth
Abstract
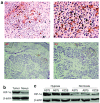
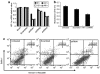
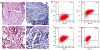
Malignant melanoma (MM) is a major public health problem. The development of effective, systemic therapies for MM is highly desired. We showed here that the transferrin receptor (TfR) was a suitable surface marker for targeting of gene therapy in MM and that the hypoxia-inducible factor-1alpha (HIF-1alpha) was an attractive therapeutic molecular target in MM. We observed that inhibition of HIF-1alpha blocked cell proliferation and induced cell apoptosis in vitro. We then showed that a transferrin-polyethylenimine-HIF-1alpha-short-hairpin RNA (Tf-PEI-HIF-1alpha-shRNA) complex could target MM specifically and efficiently both in vivo and in vitro, exploiting the high expression of the TfR in MM. The systemic delivery of sequence-specific small-interfering RNA (siRNA) against HIF-1alpha by the Tf- PEI-HIF-1alpha-shRNA complex dramatically inhibited tumor growth in the A375 MM xenograft model. The underlying concept of transfecting a HIF-1alpha shRNA expression vector complexed with Tf-PEI to block HIF-1alpha holds promise as a clinical approach to gene therapy for MM.
Figures



References
-
- Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, et al. Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc. 2007;82:364–380. - PubMed
-
- Becker JC, Kirkwood JM, Agarwala SS, Dummer R, Schrama D., and , Hauschild A. Molecularly targeted therapy for melanoma: current reality and future options. Cancer. 2006;107:2317–2327. - PubMed
-
- Tao J, Tu YT, Huang CZ, Feng AP, Wu Q, Lian YJ, et al. Inhibiting the growth of malignant melanoma by blocking the expression of vascular endothelial growth factor using an RNA interference approach. Br J Dermatol. 2005;153:715–724. - PubMed
-
- Kamat CD, Green DE, Curilla S, Warnke L, Hamilton JW, Sturup S, et al. Role of HIF signaling on tumorigenesis in response to chronic low-dose arsenic administration. Toxicol Sci. 2005;86:248–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

