Comparative study of anti-hepatitis B virus RNA interference by double-stranded adeno-associated virus serotypes 7, 8, and 9
- PMID: 19066602
- PMCID: PMC2835062
- DOI: 10.1038/mt.2008.245
Comparative study of anti-hepatitis B virus RNA interference by double-stranded adeno-associated virus serotypes 7, 8, and 9
Abstract
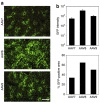
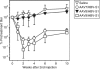
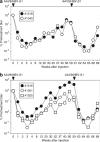
Using a hepatitis B virus (HBV) transgenic mouse model, we previously showed that a single dose of double-stranded adeno-associated virus (dsAAV) vector serotype 8 carrying a small hairpin RNA (shRNA) effectively reduces HBV replication and gene expression, but the effect gradually decreases with time. In this report, we compared the anti-HBV RNA interference (RNAi) effect of dsAAV8 with those of dsAAV7 and dsAAV9, two other hepatotropic AAV vectors, and examined whether the sequential use of these heterologous AAV vectors could prolong the anti-HBV effect. Our results showed that shRNA delivered by each of the three dsAAV vectors profoundly reduced the serum HBV titer and liver HBV mRNA and DNA levels in the transgenic mice for up to 22 weeks, with dsAAV8 having the greatest inhibitory effect, followed by dsAAV9 and dsAAV7. The potency of dsAAV8 correlated with the presence of higher levels of vector DNA and anti-HBV shRNA in the liver. An in vivo cross-administration experiment showed that preexisting anti-AAV8 antibody completely blocked the anti-HBV RNAi effect of dsAAV8, but had no effect on the potency of dsAAV7 and dsAAV9. Moreover, we demonstrated that a longer anti-HBV effect could be achieved by the sequential use of dsAAV8 and dsAAV9. These results indicate that effective and persistent HBV suppression might be achieved by a combination of the power of RNAi silencing effect and multiple treatments with different AAV serotypes.Molecular Therapy (2009) 17 2, 352-359 doi:10.1038/mt.2008.245.
Figures
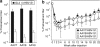



References
-
- Ganem D., and , Prince AM. Hepatitis B virus infection—natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. - PubMed
-
- Kremsdorf D, Soussan P, Paterlini-Brechot P., and , Brechot C. Hepatitis B virus-related hepatocellular carcinoma: paradigms for viral-related human carcinogenesis. Oncogene. 2006;25:3823–3833. - PubMed
-
- Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. - PubMed
-
- Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168–174. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

