Potent ligands for prokaryotic UDP-galactopyranose mutase that exploit an enzyme subsite
- PMID: 19067595
- PMCID: PMC3010353
- DOI: 10.1021/ol802094p
Potent ligands for prokaryotic UDP-galactopyranose mutase that exploit an enzyme subsite
Abstract
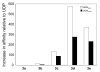
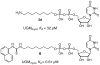
UDP-galactopyranose mutase (UGM or Glf), which catalyzes the interconversion of UDP-galactopyranose and UDP-galactofuranose, is implicated in the viability and virulence of multiple pathogenic microorganisms. Here we report the synthesis of high-affinity ligands for UGM homologues from Klebsiella pneumoniae and Mycobacterium tuberculosis. The potency of these compounds stems from their ability to access both the substrate binding pocket and an adjacent site.
Figures
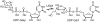

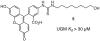
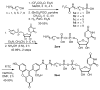
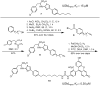
References
-
- Brennan PJ, Crick DC. Curr Top Med Chem. 2007;7:475–488. - PubMed
-
- Dykhuizen EC, May JF, Tongpenyai A, Kiessling LL. J Am Chem Soc. 2008;130:6706–6707. - PubMed
-
- Lee RE, Smith MD, Nash RJ, Griffiths RC, McNeil M, Grewal RK, Yan WX, Besra GS, Brennan PJ, Fleet GWJ. Tetrahedron Lett. 1997;38:6733–6736.
- Veerapen N, Yuan Y, Sanders DAR, Pinto BM. Carbohydr Res. 2004;339:2205–2217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

