Development of a gel permeation chromatographic assay to achieve mass balance in cellulose acetate phthalate stability studies
- PMID: 19070984
- PMCID: PMC2859192
- DOI: 10.1016/j.jpba.2008.10.039
Development of a gel permeation chromatographic assay to achieve mass balance in cellulose acetate phthalate stability studies
Abstract
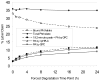
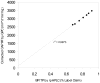
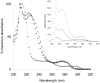
Cellulose acetate phthalate (CAP, cellulose acetate 1,2-benzenedicarboxylate) is a common polymeric oral tablet coating. CAP is also a vaginal microbicide candidate that potently inhibits HIV-1 proliferation. This paper describes the development of a precise, stability-indicating gel permeation chromatography (GPC) assay for CAP. During accelerated stability studies monitored by separate reversed-phase high performance liquid chromatography (RP-HPLC) and GPC analyses, an apparent loss of mass balance was observed. This deficit was corrected by recalculating the response factor (RF) for each degraded sample, proportional to the fraction of phthalate remaining bound to the polymeric CAP. The correction factor enabled CAP and the degradation product phthalic acid (PA) to be quantitated by a single GPC analysis. The chromatographic approach taken here could potentially apply to any polymer containing degradable chromophores.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

