Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering
- PMID: 19071168
- PMCID: PMC2698962
- DOI: 10.1016/j.jconrel.2008.10.021
Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering
Abstract
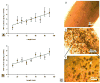
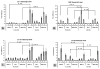
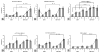
Temporally and spatially controlled delivery of growth factors in polymeric scaffolds is crucial for engineering composite tissue structures, such as osteochondral constructs. In the present study, microsphere-mediated growth factor delivery in polymer scaffolds and its impact on osteochondral differentiation of human bone marrow-derived mesenchymal stem cells (hMSCs) was evaluated. Two growth factors, bone morphogenetic protein 2 (rhBMP-2) and insulin-like growth factor I (rhIGF-I), were incorporated as a single concentration gradient or reverse gradient combining two factors in the scaffolds. To assess the gradient making system and the delivery efficiency of polylactic-co-glycolic acid (PLGA) and silk fibroin microspheres, initially an alginate gel was fabricated into a cylinder shape with microspheres incorporated as gradients. Compared to PLGA microspheres, silk microspheres were more efficient in delivering rhBMP-2, probably due to sustained release of the growth factor, while less efficient in delivering rhIGF-I, likely due to loading efficiency. The growth factor gradients formed were shallow, inducing non-gradient trends in hMSC osteochondral differentiation. Aqueous-derived silk porous scaffolds were used to incorporate silk microspheres using the same gradient process. Both growth factors formed deep and linear concentration gradients in the scaffold, as shown by enzyme-linked immunosorbent assay (ELISA). After seeding with hMSCs and culturing for 5 weeks in a medium containing osteogenic and chondrogenic components, hMSCs exhibited osteogenic and chondrogenic differentiation along the concentration gradients of rhBMP-2 in the single gradient of rhBMP-2 and reverse gradient of rhBMP-2/rhIGF-I, but not the rhIGF-I gradient system, confirming that silk microspheres were more efficient in delivering rhBMP-2 than rhIGF-I for hMSCs osteochondrogenesis. This novel silk microsphere/scaffold system offers a new option for the delivery of multiple growth factors with spatial control in a 3D culture environment for both understanding natural tissue growth process and in vitro engineering complex tissue constructs.
Figures
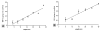

Similar articles
-
Subcritical CO2 sintering of microspheres of different polymeric materials to fabricate scaffolds for tissue engineering.Mater Sci Eng C Mater Biol Appl. 2013 Dec 1;33(8):4892-9. doi: 10.1016/j.msec.2013.08.010. Epub 2013 Aug 15. Mater Sci Eng C Mater Biol Appl. 2013. PMID: 24094202 Free PMC article.
-
Repair of an osteochondral defect by sustained delivery of BMP-2 or TGFβ1 from a bilayered alginate-PLGA scaffold.J Tissue Eng Regen Med. 2014 Jul;8(7):521-33. doi: 10.1002/term.1549. Epub 2012 Jun 26. J Tissue Eng Regen Med. 2014. PMID: 22733683
-
Development of collagen-hydroxyapatite scaffolds incorporating PLGA and alginate microparticles for the controlled delivery of rhBMP-2 for bone tissue engineering.J Control Release. 2015 Jan 28;198:71-9. doi: 10.1016/j.jconrel.2014.11.021. Epub 2014 Dec 4. J Control Release. 2015. PMID: 25481441
-
Preparation and osteogenesis of a multiple crosslinking silk fibroin/carboxymethyl chitosan/sodium alginate composite scaffold loading with mesoporous silica/poly (lactic acid-glycolic acid) microspheres.J Biomater Appl. 2025 Jan;39(6):578-591. doi: 10.1177/08853282241281439. Epub 2024 Sep 12. J Biomater Appl. 2025. PMID: 39264258
-
Effect of different sintering methods on bioactivity and release of proteins from PLGA microspheres.Mater Sci Eng C Mater Biol Appl. 2013 Oct;33(7):4343-51. doi: 10.1016/j.msec.2013.06.026. Epub 2013 Jun 28. Mater Sci Eng C Mater Biol Appl. 2013. PMID: 23910352 Free PMC article.
Cited by
-
Extracellular Calcium Modulates Chondrogenic and Osteogenic Differentiation of Human Adipose-Derived Stem Cells: A Novel Approach for Osteochondral Tissue Engineering Using a Single Stem Cell Source.Tissue Eng Part A. 2015 Sep;21(17-18):2323-33. doi: 10.1089/ten.TEA.2014.0572. Epub 2015 Jul 13. Tissue Eng Part A. 2015. PMID: 26035347 Free PMC article.
-
Electrospinning technology: a promising approach for tendon-bone interface tissue engineering.RSC Adv. 2024 Aug 19;14(36):26077-26090. doi: 10.1039/d4ra04043k. eCollection 2024 Aug 16. RSC Adv. 2024. PMID: 39161449 Free PMC article. Review.
-
Development of an in vitro model to study the impact of BMP-2 on metastasis to bone.J Tissue Eng Regen Med. 2010 Dec;4(8):590-9. doi: 10.1002/term.268. Epub 2010 Sep 23. J Tissue Eng Regen Med. 2010. PMID: 20865693 Free PMC article.
-
Adipose tissue engineering for soft tissue regeneration.Tissue Eng Part B Rev. 2010 Aug;16(4):413-26. doi: 10.1089/ten.TEB.2009.0544. Tissue Eng Part B Rev. 2010. PMID: 20166810 Free PMC article. Review.
-
Cellular and Chemical Gradients to Engineer the Meniscus-to-Bone Insertion.Adv Healthc Mater. 2019 Apr;8(7):e1800806. doi: 10.1002/adhm.201800806. Epub 2018 Dec 10. Adv Healthc Mater. 2019. PMID: 30536862 Free PMC article.
References
-
- Babensee JE, McIntire LV, Mikos AG. Growth factor delivery for tissue engineering. Pharm Res. 2000;17:497–504. - PubMed
-
- Boontheekul T, Mooney DJ. Protein-based signaling systems in tissue engineering. Curr Opin Biotechnol. 2003;14:559–565. - PubMed
-
- Whang K, Tsai DC, Nam EK, Aitken M, Sprague SM, Patel PK, Healy KE. Ectopic bone formation via rhBMP-2 delivery from porous bioabsorbable polymer scaffolds. J Biomed Mater Res. 1998;42:491–499. - PubMed
-
- Uebersax L, Merkle HP, Meinel L. Insulin-like growth factor I releasing silk fibroin scaffolds induce chondrogenic differentiation of human mesenchymal stem cells. J Control Release. 2008;127:12–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

