AIDSVAX immunization induces HIV-specific CD8+ T-cell responses in high-risk, HIV-negative volunteers who subsequently acquire HIV infection
- PMID: 19071176
- PMCID: PMC2676722
- DOI: 10.1016/j.vaccine.2008.11.071
AIDSVAX immunization induces HIV-specific CD8+ T-cell responses in high-risk, HIV-negative volunteers who subsequently acquire HIV infection
Abstract
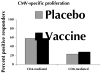
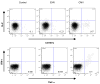
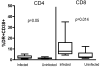
Correlates of immune protection from HIV vaccines remain undefined. The first HIV vaccine efficacy trial in the US and Europe VAX004, was designed to assess whether rgp120 envelope subunits (AIDSVAX B/B, VaxGen) can induce partial or complete protection from HIV-1 infection. No effectiveness in the reduction of either the acquisition of infection or levels of plasma viremia after HIV infection was noted. We found evidence of vaccine-specific CD8+ T cells in volunteers who received the vaccine, regardless of behavioral risk. Surprisingly, the CD8-response is significantly higher in participants who would go on to contract HIV infection. These results suggest that AIDSVAX immunization may boost preexisting immune responses-due to pre-infection exposure, and a vaccine-induced immune profile may serve as a biological marker for HIV susceptibility.
Figures

References
-
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005 Mar 1;191(5):654–65. - PubMed
-
- Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005 Mar 1;191(5):666–77. - PubMed
-
- Robinson HL. HIV/AIDS vaccines: 2007. Clinical pharmacology and therapeutics. 2007 Dec;82(6):686–93. - PubMed
-
- McMichael AJ. HIV vaccines. Annu Rev Immunol. 2006;24:227–55. - PubMed
-
- Thorner AR, Barouch DH. HIV-1 Vaccine Development: Progress and Prospects. Current infectious disease reports. 2007 Jan;9(1):71–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

