The effect of electrostatic interactions on conformational equilibria of multiply substituted tetrahydropyran oxocarbenium ions
- PMID: 19072093
- PMCID: PMC2673959
- DOI: 10.1021/jo8017846
The effect of electrostatic interactions on conformational equilibria of multiply substituted tetrahydropyran oxocarbenium ions
Abstract
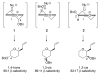
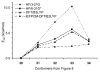
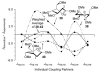
The three-dimensional structures of dioxocarbenium ions related to glycosyl cations were determined by an analysis of spectroscopic, computational, and reactivity data. Hypothetical low-energy structures of the dioxocarbenium ions were correlated with both experimentally determined (1)H NMR coupling constants and diastereoselectivity results from nucleophilic substitution reactions. This method confirmed the pseudoaxial preference of C-3 alkoxy-substituted systems and revealed the conformational preference of the C-5 alkoxymethyl group. Although the monosubstituted C-5 alkoxymethyl substituent preferred a pseudoequatorial orientation, the C-5-C-6 bond rotation was controlled by an electrostatic effect. The preferred diaxial conformer of the trans-4,5-disubstituted tetrahydropyranyl system underscored the importance of electrostatic effects in dictating conformational equilibria. In the 2-deoxymannose system, although steric effects influenced the orientation of the C-5 alkoxymethyl substituent, the all-axial conformer was favored because of electrostatic stabilization.
Figures









Similar articles
-
Structural evidence that alkoxy substituents adopt electronically preferred pseudoaxial orientations in six-membered ring dioxocarbenium ions.J Am Chem Soc. 2005 Apr 20;127(15):5322-3. doi: 10.1021/ja050830i. J Am Chem Soc. 2005. PMID: 15826161
-
Stereochemistry of nucleophilic substitution reactions depending upon substituent: evidence for electrostatic stabilization of pseudoaxial conformers of oxocarbenium ions by heteroatom substituents.J Am Chem Soc. 2003 Dec 17;125(50):15521-8. doi: 10.1021/ja037935a. J Am Chem Soc. 2003. PMID: 14664599
-
Stereoselective C-glycosylation reactions of ribose derivatives: electronic effects of five-membered ring oxocarbenium ions.J Am Chem Soc. 2005 Aug 10;127(31):10879-84. doi: 10.1021/ja0524043. J Am Chem Soc. 2005. PMID: 16076193
-
Glycosyl Oxocarbenium Ions: Structure, Conformation, Reactivity, and Interactions.Acc Chem Res. 2021 Jun 1;54(11):2552-2564. doi: 10.1021/acs.accounts.1c00021. Epub 2021 Apr 30. Acc Chem Res. 2021. PMID: 33930267 Free PMC article. Review.
-
Axial preferences in allylation reactions via the Zimmerman-Traxler transition state.Acc Chem Res. 2013 Jul 16;46(7):1659-69. doi: 10.1021/ar4000532. Epub 2013 May 14. Acc Chem Res. 2013. PMID: 23672428 Free PMC article. Review.
Cited by
-
Influence of side chain conformation and configuration on glycosyl donor reactivity and selectivity as illustrated by sialic acid donors epimeric at the 7-position.J Am Chem Soc. 2013 Dec 18;135(50):18999-9007. doi: 10.1021/ja410683y. Epub 2013 Dec 9. J Am Chem Soc. 2013. PMID: 24261615 Free PMC article.
-
Solvent effects in the nucleophilic substitutions of tetrahydropyran acetals promoted by trimethylsilyl trifluoromethanesulfonate: trichloroethylene as solvent for stereoselective C- and O-glycosylations.Org Lett. 2014 Jul 18;16(14):3684-7. doi: 10.1021/ol501471c. Epub 2014 Jul 3. Org Lett. 2014. PMID: 24991982 Free PMC article.
-
Automated Chemical Oligosaccharide Synthesis: Novel Approach to Traditional Challenges.Chem Rev. 2018 Sep 12;118(17):8105-8150. doi: 10.1021/acs.chemrev.8b00051. Epub 2018 Jun 28. Chem Rev. 2018. PMID: 29953217 Free PMC article. Review.
-
Methods for 2-Deoxyglycoside Synthesis.Chem Rev. 2018 Sep 12;118(17):7931-7985. doi: 10.1021/acs.chemrev.7b00731. Epub 2018 Jun 28. Chem Rev. 2018. PMID: 29953219 Free PMC article. Review.
-
β-Mannosylation via O-Alkylation of Anomeric Cesium Alkoxides: Mechanistic Studies and Synthesis of the Hexasaccharide Core of Complex Fucosylated N-Linked Glycans.European J Org Chem. 2020 Apr 23;2020(15):2291-2301. doi: 10.1002/ejoc.202000313. Epub 2020 Mar 19. European J Org Chem. 2020. PMID: 32431565 Free PMC article.
References
-
- Whitfield DM, Douglas SP. Glycoconjugate J. 1996;13:5–17. - PubMed
-
- Jensen HH, Pedersen CM, Bols M. Chem Eur J. 2007;13:7576–7582. - PubMed
-
- Jensen HH, Bols M. Acc Chem Res. 2006;39:259–265. - PubMed
-
- Roush WR, Sebesta DP, Bennett CE. Tetrahedron. 1997;53:8825–8836.
-
- Durham TB, Roush WR. Org Lett. 2003;5:1871–1874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

