The effect of electrostatic interactions on conformational equilibria of multiply substituted tetrahydropyran oxocarbenium ions
- PMID: 19072093
- PMCID: PMC2673959
- DOI: 10.1021/jo8017846
The effect of electrostatic interactions on conformational equilibria of multiply substituted tetrahydropyran oxocarbenium ions
Abstract
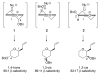
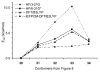
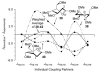
The three-dimensional structures of dioxocarbenium ions related to glycosyl cations were determined by an analysis of spectroscopic, computational, and reactivity data. Hypothetical low-energy structures of the dioxocarbenium ions were correlated with both experimentally determined (1)H NMR coupling constants and diastereoselectivity results from nucleophilic substitution reactions. This method confirmed the pseudoaxial preference of C-3 alkoxy-substituted systems and revealed the conformational preference of the C-5 alkoxymethyl group. Although the monosubstituted C-5 alkoxymethyl substituent preferred a pseudoequatorial orientation, the C-5-C-6 bond rotation was controlled by an electrostatic effect. The preferred diaxial conformer of the trans-4,5-disubstituted tetrahydropyranyl system underscored the importance of electrostatic effects in dictating conformational equilibria. In the 2-deoxymannose system, although steric effects influenced the orientation of the C-5 alkoxymethyl substituent, the all-axial conformer was favored because of electrostatic stabilization.
Figures









References
-
- Whitfield DM, Douglas SP. Glycoconjugate J. 1996;13:5–17. - PubMed
-
- Jensen HH, Pedersen CM, Bols M. Chem Eur J. 2007;13:7576–7582. - PubMed
-
- Jensen HH, Bols M. Acc Chem Res. 2006;39:259–265. - PubMed
-
- Roush WR, Sebesta DP, Bennett CE. Tetrahedron. 1997;53:8825–8836.
-
- Durham TB, Roush WR. Org Lett. 2003;5:1871–1874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

