Signaling components of redox active endosomes: the redoxosomes
- PMID: 19072143
- PMCID: PMC2842130
- DOI: 10.1089/ars.2008.2363
Signaling components of redox active endosomes: the redoxosomes
Abstract
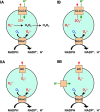
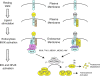
Subcellular compartmentalization of reactive oxygen species (ROS) plays a critical role in transmitting cell signals in response to environmental stimuli. In this regard, signals at the plasma membrane have been shown to trigger NADPH oxidase-dependent ROS production within the endosomal compartment and this step can be required for redox-dependent signal transduction. Unique features of redox-active signaling endosomes can include NADPH oxidase complex components (Nox1, Noxo1, Noxa1, Nox2, p47phox, p67phox, and/or Rac1), ROS processing enzymes (SOD1 and/or peroxiredoxins), chloride channels capable of mediating superoxide transport and/or membrane gradients required for Nox activity, and novel redox-dependent sensors that control Nox activity. This review will discuss the cytokine and growth factor receptors that likely mediate signaling through redox-active endosomes, and the common mechanisms whereby they act. Additionally, the review will cover ligand-independent environmental injuries, such as hypoxia/reoxygenation injury, that also appear to facilitate cell signaling through NADPH oxidase at the level of the endosome. We suggest that redox-active endosomes encompass a subset of signaling endosomes that we have termed redoxosomes. Redoxosomes are uniquely equipped with redox-processing proteins capable of transmitting ROS signals from the endosome interior to redox-sensitive effectors on the endosomal surface. In this manner, redoxosomes can control redox-dependent effector functions through the spatial and temporal regulation of ROS as second messengers.
Figures







References
-
- Abe J. Takahashi M. Ishida M. Lee JD. Berk BC. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J Biol Chem. 1997;272:20389–20394. - PubMed
-
- Ando S. Kaibuchi K. Sasaki T. Hiraoka K. Nishiyama T. Mizuno T. Asada M. Nunoi H. Matsuda I. Matsuura Y, et al. Post-translational processing of rac p21s is important both for their interaction with the GDP/GTP exchange proteins and for their activation of NADPH oxidase. J Biol Chem. 1992;267:25709–25713. - PubMed
-
- Arcaro A. Aubert M. Espinosa del Hierro ME. Khanzada UK. Angelidou S. Tetley TD. Bittermann AG. Frame MC. Seckl MJ. Critical role for lipid raft-associated Src kinases in activation of PI3K-Akt signalling. Cell Signal. 2007;19:1081–1092. - PubMed
-
- Banfi B. Clark RA. Steger K. Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

