Mechanistic studies on the copper-catalyzed N-arylation of amides
- PMID: 19072233
- PMCID: PMC2756425
- DOI: 10.1021/ja0781893
Mechanistic studies on the copper-catalyzed N-arylation of amides
Abstract
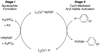
The copper-catalyzed N-arylation of amides, i.e., the Goldberg reaction, is an efficient method for the construction of products relevant to both industry and academic settings. Herein, we present mechanistic details concerning the catalytic and stoichiometric N-arylation of amides. In the context of the catalytic reaction, our findings reveal the importance of chelating diamine ligands in controlling the concentration of the active catalytic species. The consistency between the catalytic and stoichiometric results suggests that the activation of aryl halides occurs through a 1,2-diamine-ligated copper(I) amidate complex. Kinetic studies on the stoichiometric N-arylation of aryl iodides using 1,2-diamine ligated Cu(I) amidates also provide insights into the mechanism of aryl halide activation.
Figures




 ), 0.8 M (△), 0.7 M (
), 0.8 M (△), 0.7 M ( ), 0.6 M (
), 0.6 M ( ), [K3P04]o = 2.4 mmol, 2 mL of toluene, 90 °C. The black curves represent the nonlinear least-squares fit to eq. 9. The kinetic parameters are: K1 = 0.85 ± 0.05, K2 = 2.0 ± 0.2, kact = 12 ± 3 M−1•min−1.
), [K3P04]o = 2.4 mmol, 2 mL of toluene, 90 °C. The black curves represent the nonlinear least-squares fit to eq. 9. The kinetic parameters are: K1 = 0.85 ± 0.05, K2 = 2.0 ± 0.2, kact = 12 ± 3 M−1•min−1.
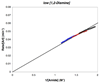
 ), 0.61 M (
), 0.61 M ( ), 0.79 M (□), [2]o = 0.83 M, [K3PO4]o = 2.4 mmol, 2 mL of toluene, 90 °C.
), 0.79 M (□), [2]o = 0.83 M, [K3PO4]o = 2.4 mmol, 2 mL of toluene, 90 °C.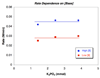





 ) [2]o = 0.94 M, (
) [2]o = 0.94 M, ( ) [2]o = 0.78 M, (□) [2]o = 0.66 M.
) [2]o = 0.78 M, (□) [2]o = 0.66 M.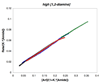
 ) [2]o = 0.8 M, [1]o = 0.8 M, (
) [2]o = 0.8 M, [1]o = 0.8 M, ( ) [2]o = 0.9 M, [1]o = 0.6 M, (
) [2]o = 0.9 M, [1]o = 0.6 M, ( ) [2]o = 0.8, [1]o = 0.6 M, (×) [2]o = 1.0 M, [1]o = 0.6 M.
) [2]o = 0.8, [1]o = 0.6 M, (×) [2]o = 1.0 M, [1]o = 0.6 M.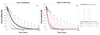



References
-
- Ullmann F. Chem. Ber. 1901;34:2174.
- Ullmann F. Chem. Ber. 1903;36:2382.
- Ullmann F, Sponagel P. Chem. Ber. 1905;38:2211.
- Goldberg I. Chem. Ber. 1906;39:1619.
-
-
For an older review highlighting the application of the Ullmann/Goldberg reaction in synthesis, see: Lindley J. Tetrahedron. 1984;40:1433.
-
-
-
For reviews on palladium-catalyzed C-N bond formation, see: Jiang L, Buchwald SL. In: Palladium-Catalyzed Aromatic Carbon-Nitrogen Bond Formation. In Metal-Catalyzed Cross-Coupling Reactions. 2nd Ed; de Meijere A, Diederich F, editors. Wiley-VCH; Weinheim: 2004. p. 699.
- Muci AR, Buchwald SL. Top. Curr. Chem. 2002;219:131.
- Hartwig JF. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E, editor. New York: Wiley-Interscience; 2002. p. 1051.
-
-
- Shen Q, Shekhar S, Stambuli JP, Hartwig JF. Angew. Chem. Int. Ed. 2005;44:1371. - PubMed
- Huang X, Anderson KW, Zim D, Jiang L, Klapars A, Buchwald SL. J. Am. Chem. Soc. 2003;125:6653. - PubMed
- Harris MC, Huang X, Buchwald SL. Org. Lett. 2002;4:2885. - PubMed
- Link JT, Sorensen B, Liu G, Pei Z, Reilly EB, Leitza S, Okasinski G. Bioorg. Med. Chem. Lett. 2001;11:973. - PubMed
- Prashad M, Hu B, Lu Y, Draper R, Har D, Repic O, Blacklock TJ. J. Org. Chem. 2000;65:2612. - PubMed
- Batch A, Dodd RH. J. Org. Chem. 1998;63:872. - PubMed
- Willoughby CA, Chapman KT. Tetrahedron Lett. 1996;37:7181.
- Ward YD, Farina V. Tetrahedron Lett. 1996;37:6993.
-
- Yin J, Buchwald SL. J. Am. Chem. Soc. 2002;124:6043. - PubMed
- Cacchi S, Fabrizi G, Goggiamani A, Zappia G. Org. Lett. 2001;3:2539. - PubMed
- Artamkina GA, Sergeev AG, Beletskaya IP. Tetrahedron. Lett. 2001;42:4381.
- Yin J, Buchwald SL. Org. Lett. 2000;2:1101. - PubMed
- Shakespeare WC. Tetrahedron. Lett. 1999;40:2035.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

