Proteomics-determined differences in the concanavalin-A-fractionated proteome of hippocampus and inferior parietal lobule in subjects with Alzheimer's disease and mild cognitive impairment: implications for progression of AD
- PMID: 19072283
- PMCID: PMC2658606
- DOI: 10.1021/pr800667a
Proteomics-determined differences in the concanavalin-A-fractionated proteome of hippocampus and inferior parietal lobule in subjects with Alzheimer's disease and mild cognitive impairment: implications for progression of AD
Abstract
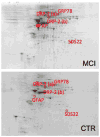
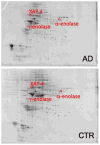
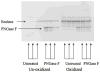
Alzheimer's disease (AD) is the most common type of dementia, comprising 60-80% of all reported cases, and currently affects 5.2 million Americans. AD is characterized pathologically by the accumulation of senile plaques (SPs), neurofibrillary tangles (NFTs), and synapse loss. The early stages of memory loss associated with AD have been studied in a condition known as amnestic mild cognitive impairment (MCI), arguably the earliest form of AD. In spite of extensive research across a variety of disciplines, the cause of AD remains elusive. Proteomics techniques have helped to advance knowledge about AD by identifying irregularities in protein expression and post-translational modifications (PTMs) in AD brain. Glycosylation is a less studied PTM with regards to AD and MCI. This PTM is important to study because glycosylation is involved in proper protein folding, protein anchoring to cell membranes, and the delivery of proteins to organelles, and these processes are impaired in AD. Concanavalin-A (Con-A) binds to N-linked glycoproteins, but hydrophobic sites on nonglycoproteins are also known to bind Con-A. To our knowledge, the present study is the first to examine Con-A-associated brain proteins in MCI and AD with focus on the hippocampus and inferior parietal lobule (IPL) brain regions. Proteins found in AD hippocampus with altered levels are glutamate dehydrogenase (GDH), glial fibrillary acidic protein (GFAP), tropomyosin 3 (TPM3), Rab GDP-dissociation inhibitor XAP-4 (XAP4), and heat shock protein 90 (HSP90). Proteins found with altered levels in AD IPL are alpha-enolase, gamma-enolase, and XAP-4. MCI hippocampal proteins with altered levels are dihydropyrimidase-2 (DRP2), glucose-regulated protein 78 (GRP-78), protein phosphatase related protein Sds-22 (Sds22), and GFAP and the only protein found with altered levels in MCI IPL was beta-synuclein. These results are discussed with reference to biochemical and pathological alterations in and progression of AD.
Figures




References
-
- 2008 Alzheimer’s disease facts and figures. Alzheimers Dement. 2008;4(2):110–33. - PubMed
-
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120(3):885–90. - PubMed
-
- Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol. 2004;6(11):1054–61. - PubMed
-
- Selkoe DJ. Cell biology of the amyloid beta-protein precursor and the mechanism of Alzheimer’s disease. Annu Rev Cell Biol. 1994;10:373–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

