Marrow cell infusion attenuates vascular remodeling in a murine model of monocrotaline-induced pulmonary hypertension
- PMID: 19072290
- PMCID: PMC3135187
- DOI: 10.1089/scd.2008.0237
Marrow cell infusion attenuates vascular remodeling in a murine model of monocrotaline-induced pulmonary hypertension
Abstract
There have been reports of marrow cells converting into pulmonary epithelial cells after marrow transplantation in irradiated mice. We evaluated the impact of whole bone marrow (WBM) infusion in mice, with or without total body irradiation (TBI), treated with saline or monocrotaline (MCT), which induces pulmonary hypertension (PH). C57BL/6 mice were injected with MCT or saline weekly for 4 weeks. Cohorts were then infused with saline vehicle (vehicle) or WBM from C57BL/-Tg(UBC-GFP)30Scha/J mice, with or without previous TBI (WBM or WBM/TBI). Four weeks later, right ventricular peak pressures (RVPP), right ventricular free wall-to-body weight ratios (RV/BW), and pulmonary vessel wall thickness-to-blood vessel diameter ratios (PVWT/D) were determined. WBM infusion and WBM following TBI induced increases in RVPP and RV/BW in saline-treated mice, while only TBI-exposed mice showed additional increases in PVWT/D. MCT increased RVPP, RV/BW, and PVWT/D in mice given vehicle or WBM alone, but not in mice given WBM/TBI. RVPP and RV/BW were not significantly lower in MCT mice given WBM/TBI than in MCT mice treated with vehicle, but MCT-treated mice given WBM or TBI/WBM had significantly lower PVWT/D compared to MCT-treated mice given saline vehicle. No donor WBM-derived pulmonary vascular cells were detected, suggesting that the observed effects of WBM infusion may be due to paracrine effects separate from cell conversions. The observation of PH after marrow infusion suggests an additional mechanism for lung toxicity seen in marrow transplantation. In conclusion, WBM alone appears to increase RVPP and RV/BW in normal mice but the combination of WBM and TBI attenuates MCT-induced PH.
Figures


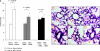

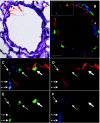
References
-
- Aliotta JM. Keaney P. Passero M. Dooner MS. Pimentel J. Greer D. Demers D. Foster B. Peterson A. Dooner G. Theise ND. Abedi M. Colvin GA. Quesenberry PJ. Bone marrow production of lung cells: the impact of G-CSF, cardiotoxin, graded doses of irradiation and subpopulation phenotype. Exp Hematol. 2008;34:230–241. - PMC - PubMed
-
- Theise ND. Henegariu O. Grove J. Jagirdar J. Kao PN. Crawford JM. Badve S. Saxena R. Krause DS. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol. 2008;30:1333–1338. - PubMed
-
- Herzog E. Van Arnam J. Hu B. Krause DS. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells. 2008;24:1986–1992. - PubMed
-
- Abe S. Lauby G. Boyer C. Rennard SI. Sharp JG. Transplanted BM and BM side population cells contribute progeny to the lung and liver in irradiated mice. Cytotherapy. 2008;5:623–633. - PubMed
-
- Grove JE. Lutzko C. Priller J. Henegariu O. Theise ND. Kohn DB. Krause DS. Marrow-derived cells as vehicles for delivery of gene therapy to pulmonary epithelium. Am J Respir Cell Mol Biol. 2008;27:645–651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

