Surface confined metallosupramolecular architectures: formation and scanning tunneling microscopy characterization
- PMID: 19072706
- PMCID: PMC2654282
- DOI: 10.1021/ar800117j
Surface confined metallosupramolecular architectures: formation and scanning tunneling microscopy characterization
Abstract
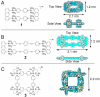
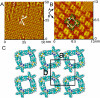
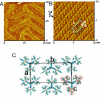
Metallosupramolecular compounds have attracted a great deal of attention over the past two decades largely because of their unique, highly complex structural characteristics and their potential electronic, magnetic, optical, and catalytic properties. These molecules can be prepared with relative ease using coordination-driven self-assembly techniques. In particular, the use of electron-poor square-planar Pt(II) transition metals in conjunction with rigid, electron-rich pyridyl donors has enabled the spontaneous self-assembly of a rich library of 2D metallacyclic and 3D metallacage assemblies via the directional-bonding approach. With this progress in the preparation and characterization of metallosupramolecules, researchers have now turned their attention toward fully exploring and developing their materials properties. Assembling metallosupramolecular compounds on solid supports represents a vitally important step toward developing their materials properties. Surfaces provide a means of uniformly aligning and orienting these highly symmetric metallacycles and metallacages. This uniformity increases the level of coherence between molecules above that which can be achieved in the solution phase and provides a way to integrate adsorbed layers, or adlayers, into a solid-state materials setting. The dynamic nature of kinetically labile Pt(II)-N coordination bonds requires us to adjust deposition and imaging conditions to retain the assemblies' stability. Toward these aims, we have used scanning tunneling microscopy (STM) to image these adlayers and to understand the factors that govern surface self-assembly and the interactions that influence their structure and stability. This Account describes our efforts to deposit 2D rectangular and square metallacycles and 3D trigonal bipyramidal and chiral trigonal prism metallacages on highly oriented pyrolytic graphite (HOPG) and Au(111) substrates to give intact assemblies and ordered adlayers. We have investigated the effects of varying the size, symmetry, and dimensionality of supramolecular adsorbates, the choice of substrate, the use of a molecular template, and the effects of chirality. Our systematic investigations provide insights into the various adsorbate-adsorbate and substrate-adsorbate interactions that largely determine the architecture of each assembly and affect their performance in a materials setting. Rational control over adlayer formation and structure will greatly enhance the potential of these supramolecules to be used in a variety of applications such as host-guest sensing/diagnostic systems, molecular electronic devices, and heterogeneous stereoselective synthesis and catalysis.
Figures





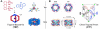


References
-
-
See, for example: Kay ER, Leigh DA, Zerbetto F. Synthetic Molecular Motors and Mechanical Machines. Angew. Chem. Int. Ed. 2007;46:72–191.
-
-
- Lathan AH, Williams ME. Controlling Transport and Chemical Functionality of Magnetic Nanoparticles. Acc. Chem. Res. 2008;41:411–420. - PubMed
- Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean A, Han MS, Mirkin CA. Oligonucleotide-Modified Gold Nanoparticles for Intracellular Gene Regulation. Science. 2006;312:1027–1030. - PubMed
-
- Barlow SM, Raval R. Complex Organic Molecules at Metal Surfaces: Bonding, Organization and Chirality. Surface Science Reports. 2003;50:201–341. and references therein.
-
-
See, for example: van Delden RA, ter Wiel MKJ, Pollard MM, Vicario J, Koumura N, Feringa BL. Undirectional Molecular Motor on a Gold Surface. Nature. 2005;437:1337.
-
-
- Gates BG, Xu Q, Stewart M, Ryan D, Wilson CG, Whitesides GM. New Approaches to Nanofabrication: Molding, Printing, and Other Techniques. Chem. Rev. 2005;105:1171–1196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

