Tumor-derived microvesicles in sera of patients with head and neck cancer and their role in tumor progression
- PMID: 19073006
- PMCID: PMC2647573
- DOI: 10.1002/hed.20968
Tumor-derived microvesicles in sera of patients with head and neck cancer and their role in tumor progression
Abstract
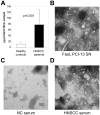
Background: Tumor-derived membranous vesicles (MV) isolated from sera of the patients with squamous cell carcinomas of the head and neck (HNSCC) induce apoptosis of activated CD8(+) T cells. We tested if MV molecular profile and activity correlate with disease progression.
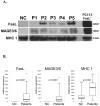
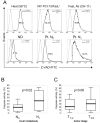
Methods: CD8(+) Jurkat cells were incubated with MAGE 3/6(+), FasL(+), MHC class I(+) MV isolated from sera of 60 patients with HNSCC and 25 normal controls by exclusion chromatography and ultracentrifugation. Z-VAD-FITC binding to Jurkat was measured and correlated with clinical data.
Results: MV from patients' sera, but not from sera of normal controls, induced Jurkat cell apoptosis. Forty-five percent T cells+MV from patients with N(1)-N(3) disease were apoptotic versus 18% T cells+MV from patients with N(0) disease (p < .008). MV from patients with active disease (AD) expressed higher FasL levels than MV from patients with no evident disease (NED) or normal controls (p <or= .01).
Conclusion: MAGE 3/6(+), FasL(+), and MHCI(+) MV in sera of patients induced T-cell apoptosis, which correlated with disease activity and the presence of lymph node metastases.
Figures

References
-
- Young MR. Protective mechanisms of head and neck squamous cell carcinomas from immune assault. Head Neck. 2006;28:462–470. - PubMed
-
- Grandis JR, Falkner DM, Melhem MF, Gooding WE, Drenning SD, Morel PA. Human leukocyte antigen class I allelic and haplotype loss in squamous cell carcinoma of the head and neck: clinical and immunogenetic consequences. Clin Cancer Res. 2000;6:2794–2802. - PubMed
-
- Whiteside TL. Immunobiology of head and neck cancer. Cancer Metastasis Rev. 2005;24:95–105. - PubMed
-
- Kim JW, Tsukishiro T, Johnson JT, Whiteside TL. Expression of pro- and antiapoptotic proteins in circulating CD8+ T cells of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10:5101–5110. - PubMed
-
- Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

