The human placenta is a hematopoietic organ during the embryonic and fetal periods of development
- PMID: 19073167
- PMCID: PMC2668662
- DOI: 10.1016/j.ydbio.2008.11.017
The human placenta is a hematopoietic organ during the embryonic and fetal periods of development
Retraction in
-
Retraction notice to "The human placenta is a hematopoietic organ during the embryonic and fetal periods of development" [Dev. Biol. 327 (2009) 24–33].Dev Biol. 2014 Apr 15;388(2):216. doi: 10.1016/j.ydbio.2014.03.001. Dev Biol. 2014. PMID: 24772463 Free PMC article. No abstract available.
Abstract
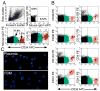
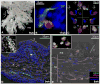
We studied the potential role of the human placenta as a hematopoietic organ during embryonic and fetal development. Placental samples contained two cell populations-CD34(++)CD45(low) and CD34(+)CD45(low)-that were found in chorionic villi and in the chorioamniotic membrane. CD34(++)CD45(low) cells express many cell surface antigens found on multipotent primitive hematopoietic progenitors and hematopoietic stem cells. CD34(++)CD45(low) cells contained colony-forming units culture (CFU-C) with myeloid and erythroid potential in clonogenic in vitro assays, and they generated CD56(+) natural killer cells and CD19(+)CD20(+)sIgM(+) B cells in polyclonal liquid cultures. CD34(+)CD45(low) cells mostly comprised erythroid- and myeloid-committed progenitors, while CD34(-) cells lacked CFU-C. The placenta-derived precursors were fetal in origin, as demonstrated by FISH using repeat-sequence chromosome-specific probes for X and Y. The number of CD34(++)CD45(low) cells increased with gestational age, but their density (cells per gram of tissue) peaked at 5-8 wk, decreasing more than sevenfold at the onset of the fetal phase (9 wk of gestation). In addition to multipotent progenitors, the placenta contained myeloid- and erythroid-committed progenitors indicative of active in situ hematopoiesis. These data suggest that the human placenta is an important hematopoietic organ, raising the possibility of banking placental hematopoietic stem cells along with cord blood for transplantation.
Conflict of interest statement
Figures





References
-
- Alvarez-Silva M, Belo-Diabangouaya P, Salaun J, Dieterlen-Lievre F. Mouse placenta is a major hematopoietic organ. Development. 2003;130:5437–5444. - PubMed
-
- Bárcena A, Muench MO, Galy AHM, Cupp J, Roncarolo MG, Phillips JH, Spits H. Phenotypic and functional analysis of T-cell precursors in the human fetal liver and thymus. CD7 expression in the early stages of T-and myeloid-cell development. Blood. 1993;82:3401–3414. - PubMed
-
- Bárcena A, Muench MO, Song KS, Ohkubo T, Harrison MR. Role of CD95/Fas and its ligand in the regulation of the growth of human CD34++CD38− fetal liver cells. Exp Hematol. 1999;27:1428–1439. - PubMed
-
- Bárcena A, Park SW, Banapour B, Muench MO, Mechetner EB. Expression of Fas/CD95 and bcl-2 in primitive hematopoietic progenitors in the human fetal liver. Blood. 1996;88:2013–2025. - PubMed
-
- Benischke K, Kaufmann P. Pathology of the Human Placenta. Springer; New York: 2000. Hofbauer cells; pp. 81–85.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

