Selective blockade of PGE2 EP1 receptor protects brain against experimental ischemia and excitotoxicity, and hippocampal slice cultures against oxygen-glucose deprivation
- PMID: 19073437
- PMCID: PMC6015740
- DOI: 10.1007/BF03033858
Selective blockade of PGE2 EP1 receptor protects brain against experimental ischemia and excitotoxicity, and hippocampal slice cultures against oxygen-glucose deprivation
Abstract
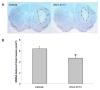
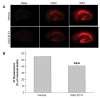
Cyclooxygenase-2 (COX-2) enzyme increases abnormally during excitotoxicity and cerebral ischemia and promotes neurotoxicity. Although COX-2 inhibitors could be beneficial, they have significant side effects. We and others have shown that the EP1 receptor is important in mediating PGE2 toxicity. Here, we tested the hypothesis that pretreatment with a highly selectiveEP1 receptor antagonist, ONO-8713, would improve stroke outcome and that post-treatment would attenuate NMDA-induced acute excitotoxicity and protect organotypic brain slices from oxygen-glucose deprivation (OGD)-induced toxicity. Male C57BL/6 mice were injected intracerebroventricularly with ONO-8713 before being subjected to 90-min middle cerebral artery occlusion (MCAO) and 96-h reperfusion.Significant reduction in infarct size was observed in groups given 0.1 (25.9 +/- 4.7%) and 1.0 nmol(27.7 +/- 2.8%) ONO-8713 as compared with the vehicle-treated control group. To determine the effects of ONO-8713 post-treatment on NMDA induced excitotoxicity, mice were given a unilateral intrastriatal NMDA injection followed by one intraperitoneal injection of 10 microg/kg ONO-8713, 1 and 6 h later. Significant attenuation of brain damage (26.6 +/-4.9%) was observed at 48 hin the ONO-8713-treated group. Finally, brain slice cultures were protected (25.5 +/- 2.9%) by the addition of ONO-8713 to the medium after OGD.These findings support the notion that the EP1receptor propagates neurotoxicity and that selective blockade could be considered as a potential preventive and/or therapeutic tool against ischemic/hypoxic neurological conditions.
Conflict of interest statement
There are no conflicts of interest with this work.
Figures

References
-
- T Abe, Kunz A, Shimamura M, Zhou P, Anrather J, Iadecola C. The neuroprotective effect of prostaglandin E2 EP1 receptor inhibition has a wide therapeutic window, is sustained in time and is not sexually dimorphic. J Cereb Blood Flow Metab. 2008 doi: 10.1038/jcbfm.2008.88. In press. - DOI - PMC - PubMed
-
- Adams J, Collaco-Moraes Y, de Belleroche J. Cyclooxygenase-2 induction in cerebral cortex: an intracellular response to synaptic excitation. J Neurochem. 1996;66:6–13. - PubMed
-
- Ahmad AS, Zhuang H, Echeverria V, Doré S. Stimulation of prostaglandin EP2 receptors prevents NMDA-induced excitotoxicity. J Neurotrauma. 2006a;23:1895–1903. - PubMed
-
- Ahmad AS, Saleem S, Ahmad M, Doré S. Prostaglandin EP1 receptor contributes to excitotoxicity and focal ischemic brain damage. Toxicol Sci. 2006b;89:265–270. - PubMed
-
- Ahmad M, Saleem S, Zhuang H, Ahmad AS, Echeverria V, Sapirstein A, Doré S. 1-HydroxyPGE1 reduces infarction volume in mouse transient cerebral ischemia. Eur. J Neurosci. 2006c;23:35–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

