A transient outward potassium current activator recapitulates the electrocardiographic manifestations of Brugada syndrome
- PMID: 19073629
- PMCID: PMC2642600
- DOI: 10.1093/cvr/cvn339
A transient outward potassium current activator recapitulates the electrocardiographic manifestations of Brugada syndrome
Abstract
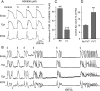
Aims: Transient outward potassium current (I(to)) is thought to be central to the pathogenesis of the Brugada syndrome (BrS). However, an I((to)) activator has not been available with which to validate this hypothesis. Here, we provide a direct test of the hypothesis using a novel I(to) activator, NS5806.
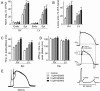
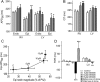
Methods and results: Isolated canine ventricular myocytes and coronary-perfused wedge preparations were used. Whole-cell patch-clamp studies showed that NS5806 (10 microM) increased peak I(to) at +40 mV by 79 +/- 4% (24.5 +/- 2.2 to 43.6 +/- 3.4 pA/pF, n = 7) and slowed the time constant of inactivation from 12.6 +/- 3.2 to 20.3 +/- 2.9 ms (n = 7). The total charge carried by I(to) increased by 186% (from 363.9 +/- 40.0 to 1042.0 +/- 103.5 pA x ms/pF, n = 7). In ventricular wedge preparations, NS5806 increased phase 1 and notch amplitude of the action potential in the epicardium, but not in the endocardium, and accentuated the ECG J-wave, leading to the development of phase 2 re-entry and polymorphic ventricular tachycardia (n = 9). Although sodium and calcium channel blockers are capable of inducing BrS only in right ventricular (RV) wedge preparations, the I(to) activator was able to induce the phenotype in wedges from both ventricles. NS5806 induced BrS in 4/6 right and 2/10 left ventricular wedge preparations.
Conclusion: The I(to) activator NS5806 recapitulates the electrographic and arrhythmic manifestation of BrS, providing evidence in support of its pivotal role in the genesis of the disease. Our findings also suggest that a genetic defect leading to a gain of function of I(to) could explain variants of BrS, in which ST-segment elevation or J-waves are evident in both right and left ECG leads.
Figures
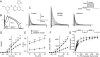
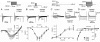


Comment in
-
Arrhythmogenic Brugada syndrome substrate: a proof of principle.Cardiovasc Res. 2009 Mar 1;81(4):635-6. doi: 10.1093/cvr/cvp008. Epub 2009 Jan 9. Cardiovasc Res. 2009. PMID: 19136529 No abstract available.
References
-
- Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. - PubMed
-
- Horigome H, Shigeta O, Kuga K, Isobe T, Sakakibara Y, Yamaguchi I, et al. Ventricular fibrillation during anesthesia in association with J waves in the left precordial leads in a child with coarctation of the aorta. J Electrocardiol. 2003;36:339–343. - PubMed
-
- Ogawa M, Kumagai K, Yamanouchi Y, Saku K. Spontaneous onset of ventricular fibrillation in Brugada syndrome with J wave and ST-segment elevation in the inferior leads. Heart Rhythm. 2005;2:97–99. - PubMed
-
- Potet F, Mabo P, Le CG, Probst V, Schott JJ, Airaud F, et al. Novel Brugada SCN5A mutation leading to ST segment elevation in the inferior or the right precordial leads. J Cardiovasc Electrophysiol. 2003;14:200–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

