The Na+-Pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary Pi
- PMID: 19073637
- PMCID: PMC2670642
- DOI: 10.1152/ajprenal.90623.2008
The Na+-Pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary Pi
Abstract
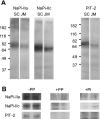
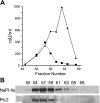
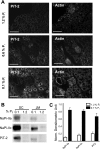
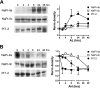
The principal mediators of renal phosphate (P(i)) reabsorption are the SLC34 family proteins NaPi-IIa and NaPi-IIc, localized to the proximal tubule (PT) apical membrane. Their abundance is regulated by circulatory factors and dietary P(i). Although their physiological importance has been confirmed in knockout animal studies, significant P(i) reabsorptive capacity remains, which suggests the involvement of other secondary-active P(i) transporters along the nephron. Here we show that a member of the SLC20 gene family (PiT-2) is localized to the brush-border membrane (BBM) of the PT epithelia and that its abundance, confirmed by Western blot and immunohistochemistry of rat kidney slices, is regulated by dietary P(i). In rats treated chronically on a high-P(i) (1.2%) diet, there was a marked decrease in the apparent abundance of PiT-2 protein in kidney slices compared with those from rats kept on a chronic low-P(i) (0.1%) diet. In Western blots of BBM from rats that were switched from a chronic low- to high-P(i) diet, NaPi-IIa showed rapid downregulation after 2 h; PiT-2 was also significantly downregulated at 24 h and NaPi-IIc after 48 h. For the converse dietary regime, NaPi-IIa showed adaptation within 8 h, whereas PiT-2 and NaPi-IIc showed a slower adaptive trend. Our findings suggest that PiT-2, until now considered as a ubiquitously expressed P(i) housekeeping transporter, is a novel mediator of P(i) reabsorption in the PT under conditions of acute P(i) deprivation, but with a different adaptive time course from NaPi-IIa and NaPi-IIc.
Figures

Comment in
-
PiT-2 coming out of the pits.Am J Physiol Renal Physiol. 2009 Apr;296(4):F689-90. doi: 10.1152/ajprenal.00007.2009. Epub 2009 Feb 4. Am J Physiol Renal Physiol. 2009. PMID: 19193727 No abstract available.
References
-
- Bai L, Collins JF, Ghishan FK. Cloning and characterization of a type III Na-dependent phosphate cotransporter from mouse intestine. Am J Physiol Cell Physiol 279: C1135–C1143, 2000. - PubMed
-
- Berndt T, Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Annu Rev Physiol 69: 341–359, 2007. - PubMed
-
- Berndt TJ, Kumar R. Clinical disturbances of phosphate homeostasis. In: Seldin and Giebisch's The Kidney, edited by Alpern RJ and Hebert SC. New York, NY: Academic, 2008, p. 1989–2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

