Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes
- PMID: 19073721
- PMCID: PMC2643732
- DOI: 10.1128/JVI.01699-08
Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes
Abstract
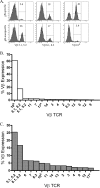
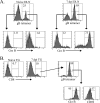
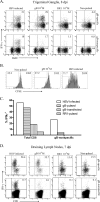
In C57BL/6 (B6) mice, most herpes simplex virus (HSV)-specific CD8 T cells recognize a strongly immunodominant epitope on glycoprotein B (gB498) and can inhibit HSV type 1 (HSV-1) reactivation from latency in trigeminal ganglia (TG). However, half of the CD8 T cells retained in latently infected TG of B6 mice are not gB498 specific and have been largely ignored. The following observations from our current study indicate that these gB498-nonspecific CD8 T cells are HSV specific and may contribute to the control of HSV-1 latency. First, following corneal infection, OVA257-specific OT-1 CD8 T cells do not infiltrate the infected TG unless mice are simultaneously immunized with OVA257 peptide, and then they are not retained. Second, 30% of CD8 T cells in acutely infected TG that produce gamma interferon in response to HSV-1 stimulation directly ex vivo are gB498 nonspecific, and these cells maintain an activation phenotype during viral latency. Finally, gB498-nonspecific CD8 T cells are expanded in ex vivo cultures of latently infected TG and inhibit HSV-1 reactivation from latency in the absence of gB498-specific CD8 T cells. We conclude that many of the CD8 T cells that infiltrate and are retained in infected TG are HSV specific and potentially contribute to maintenance of HSV-1 latency. Identification of the viral proteins recognized by these cells will contribute to a better understanding of the dynamics of HSV-1 latency.
Figures
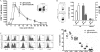



Similar articles
-
Influence of an immunodominant herpes simplex virus type 1 CD8+ T cell epitope on the target hierarchy and function of subdominant CD8+ T cells.PLoS Pathog. 2017 Dec 4;13(12):e1006732. doi: 10.1371/journal.ppat.1006732. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29206240 Free PMC article.
-
Herpes Simplex Virus 1-Specific CD8+ T Cell Priming and Latent Ganglionic Retention Are Shaped by Viral Epitope Promoter Kinetics.J Virol. 2020 Feb 14;94(5):e01193-19. doi: 10.1128/JVI.01193-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31826989 Free PMC article.
-
Delaying the expression of herpes simplex virus type 1 glycoprotein B (gB) to a true late gene alters neurovirulence and inhibits the gB-CD8+ T-cell response in the trigeminal ganglion.J Virol. 2010 Sep;84(17):8811-20. doi: 10.1128/JVI.00496-10. Epub 2010 Jun 23. J Virol. 2010. PMID: 20573821 Free PMC article.
-
CD8+ T cells patrol HSV-1-infected trigeminal ganglia and prevent viral reactivation.J Neurovirol. 2011 Dec;17(6):528-34. doi: 10.1007/s13365-011-0062-1. Epub 2011 Dec 8. J Neurovirol. 2011. PMID: 22161682 Review.
-
Control of HSV-1 latency in human trigeminal ganglia--current overview.J Neurovirol. 2011 Dec;17(6):518-27. doi: 10.1007/s13365-011-0063-0. Epub 2011 Dec 3. J Neurovirol. 2011. PMID: 22139603 Review.
Cited by
-
PD-L1/B7-H1 regulates the survival but not the function of CD8+ T cells in herpes simplex virus type 1 latently infected trigeminal ganglia.J Immunol. 2013 Jun 15;190(12):6277-86. doi: 10.4049/jimmunol.1300582. Epub 2013 May 8. J Immunol. 2013. PMID: 23656736 Free PMC article.
-
Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates?Vaccine. 2011 Aug 11;29(35):5824-36. doi: 10.1016/j.vaccine.2011.06.083. Epub 2011 Jun 28. Vaccine. 2011. PMID: 21718746 Free PMC article. Review.
-
Herpesvirus-Associated Lymphadenitis Distorts Fibroblastic Reticular Cell Microarchitecture and Attenuates CD8 T Cell Responses to Neurotropic Infection in Mice Lacking the STING-IFNα/β Defense Pathways.J Immunol. 2016 Sep 15;197(6):2338-52. doi: 10.4049/jimmunol.1600574. Epub 2016 Aug 10. J Immunol. 2016. PMID: 27511736 Free PMC article.
-
Herpes keratitis.Prog Retin Eye Res. 2013 Jan;32:88-101. doi: 10.1016/j.preteyeres.2012.08.002. Epub 2012 Aug 27. Prog Retin Eye Res. 2013. PMID: 22944008 Free PMC article. Review.
-
Defining the herpes simplex virus-specific CD8+ T cell repertoire in C57BL/6 mice.J Immunol. 2011 Apr 1;186(7):3927-33. doi: 10.4049/jimmunol.1003735. Epub 2011 Feb 25. J Immunol. 2011. PMID: 21357536 Free PMC article.
References
-
- Allan, R. S., J. Waithman, S. Bedoui, C. M. Jones, J. A. Villadangos, Y. Zhan, A. M. Lew, K. Shortman, W. R. Heath, and F. R. Carbone. 2006. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25153-162. - PubMed
-
- Baron, C., M. C. Meunier, E. Caron, C. Cote, M. J. Cameron, D. J. Kelvin, R. LeBlanc, V. Rineau, and C. Perreault. 2006. Asynchronous differentiation of CD8 T cells that recognize dominant and cryptic antigens. J. Immunol. 1778466-8475. - PubMed
-
- Coles, R. M., S. N. Mueller, W. R. Heath, F. R. Carbone, and A. G. Brooks. 2002. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J. Immunol. 168834-838. - PubMed
-
- Coulombe, M., and R. G. Gill. 1996. T lymphocyte indifference to extrathymic islet allografts. J. Immunol. 1561998-2003. - PubMed
-
- Decman, V., M. L. Freeman, P. R. Kinchington, and R. L. Hendricks. 2005. Immune control of HSV-1 latency. Viral Immunol. 18466-473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

