Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance
- PMID: 19073774
- PMCID: PMC2646053
- DOI: 10.2337/db08-1078
Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance
Abstract
Objective: Skeletal muscle insulin resistance is associated with lipid accumulation, but whether insulin resistance is due to reduced or enhanced flux of long-chain fatty acids into the mitochondria is both controversial and unclear. We hypothesized that skeletal muscle-specific overexpression of the muscle isoform of carnitine palmitoyltransferase 1 (CPT1), the enzyme that controls the entry of long-chain fatty acyl CoA into mitochondria, would enhance rates of fatty acid oxidation and improve insulin action in muscle in high-fat diet insulin-resistant rats.
Research design and methods: Rats were fed a standard (chow) or high-fat diet for 4 weeks. After 3 weeks, in vivo electrotransfer was used to overexpress the muscle isoform of CPT1 in the distal hindlimb muscles (tibialis anterior and extensor digitorum longus [EDL]). Skeletal muscle insulin action was examined in vivo during a hyperinsulinemic-euglycemic clamp.
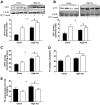
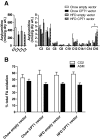
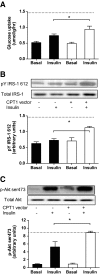
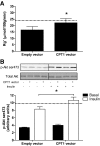
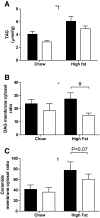
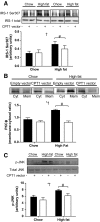
Results: In vivo electrotransfer produced a physiologically relevant increase of approximately 20% in enzyme activity; and although the high-fat diet produced insulin resistance in the sham-treated muscle, insulin action was improved in the CPT1-overexpressing muscle. This improvement was associated with a reduction in triacylglycerol content, the membrane-to-cytosolic ratio of diacylglycerol, and protein kinase C theta activity. Importantly, overexpression of CPT1 did not affect markers of mitochondrial capacity or function, nor did it alter skeletal muscle acylcarnitine profiles irrespective of diet.
Conclusions: Our data provide clear evidence that a physiological increase in the capacity of long-chain fatty acyl CoA entry into mitochondria is sufficient to ameliorate lipid-induced insulin resistance in muscle.
Figures
References
-
- Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH: Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46: 983–988, 1997 - PubMed
-
- Itani SI, Ruderman NB, Schmieder F, Boden G: Lipid-induced insulin resistance in human muscle is associated with changes in diacylglyceol, protein kinase C, and IκB-α. Diabetes 51: 2005–2011, 2002 - PubMed
-
- Adams JM II, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ: Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53: 25–31, 2004 - PubMed
-
- Levin MC, Monetti M, Watt MJ, Sajan MP, Stevens RD, Bain JR, Newgard CB, Farese RV Sr, Farese RV Jr: Increased lipid accumulation and insulin resistance in transgenic mice expressing DGAT2 in glycolytic (type II) muscle. Am J Physiol Endocrinol Metab 293: E1772–E1781, 2008 - PubMed
-
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA: Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5: 167–179, 2007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

