Phase I trial of rosiglitazone in FSGS: I. Report of the FONT Study Group
- PMID: 19073787
- PMCID: PMC2615712
- DOI: 10.2215/CJN.02310508
Phase I trial of rosiglitazone in FSGS: I. Report of the FONT Study Group
Abstract
Background and objectives: Patients with primary focal segmental glomerulosclerosis (FSGS) who are resistant to standard therapy are at high risk for progressive chronic kidney disease. Prevention of renal fibrosis represents a promising strategy to slow or halt kidney function decline. This paper presents the results of a Phase I clinical trial of rosiglitazone, a thiazolidinedione, that exerts antifibrotic effects in animal models of FSGS. The primary goal was assessment of safety, tolerability, and pharmacokinetics (PK) of rosiglitazone.
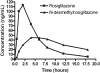
Design, setting, participants, & measurements: Eleven patients, including eight boys/men and three girls/women, with mean age 15 +/- 6 yr and estimated GFR 131 +/- 62 ml/min/1.73 m(2), received rosiglitazone, 3 mg/m(2)/d for 16 wk. PK was assessed twice, after the initial dose and after attaining steady state, in a General Clinical Research Center.
Results: There were no serious adverse events or cardiovascular complications. Rosiglitazone was well tolerated by all patients, as judged by the Treatment Satisfaction Questionnaire for Medication. The PK studies indicated that the area under the curve was decreased by 40 to 50% and oral clearance of rosiglitazone was increased by 250 to 300% in patients with resistant FSGS compared with healthy controls and patients with nonproteinuric stage 2 chronic kidney disease.
Conclusions: Rosiglitazone therapy was safe and well tolerated. PK assessment of potential novel therapies for resistant FSGS is necessary to define appropriate dosing regimens. There is rationale to evaluate the efficacy of rosiglitazone as an antifibrotic agent for resistant FSGS in Phase II/III clinical trials.
Figures

References
-
- Weissgarten J, Berman S, Efrati S, et al: Apoptosis and proliferation of cultured mesangial cells isolated from kidneys of rosiglitazone-treated pregnant diabetic rats. Nephrol Dial Transplant 21: 1198–1204, 2006 - PubMed
-
- Panchapakesan U, Sumual S, Pollock CA, et al: PPAR-γ agonists exert antifibrotic effects in renal tubular cells exposed to high glucose. Am J Physiol Renal Physiol 289: F1153–F1158, 2005 - PubMed
-
- Zafiriou S, Stanners SR, Saad S, et al: Pioglitazone inhibits cell growth and reduces matrix production in human kidney fibroblasts. J Am Soc Nephrol 16: 638–645, 2005 - PubMed
-
- Yang HC, Ma LJ, Ma J, Fogo AB Peroxisome proliferator-activated receptor-gamma agonist is protective in podocyte injury-associated sclerosis. Kidney Int 69: 1756–1764, 2006 - PubMed
-
- Bakris G, Viberti G, Weston WM, Heise M, Porter LE, Freed MI:Rosiglitazone reduces urinary albumin excretion in type II diabetes. J Hum Hypertens 17: 7–12, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

