C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis
- PMID: 19073822
- PMCID: PMC2637051
- DOI: 10.1681/ASN.2008050497
C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis
Abstract
Anti-neutrophil cytoplasmic autoantibody (ANCA)-induced necrotizing crescentic glomerulonephritis (NCGN) requires complement participation in its pathogenesis. We tested the hypothesis that the anaphylatoxin C5a is pivotal to disease induction via the neutrophil C5a receptor (C5aR). Supernatants from ANCA-activated neutrophils activated the complement cascade in normal serum, producing C5a. This conditioned serum primed neutrophils for ANCA-induced respiratory burst; neutrophil C5aR blockade abrogated this priming, but C3aR blockade did not. Furthermore, recombinant C5a but not C3a dosage-dependently primed neutrophils for ANCA-induced respiratory burst. To test the role of C5aR in a model of NCGN, we immunized myeloperoxidase-deficient mice with myeloperoxidase, irradiated them, and transplanted bone marrow from wild-type mice or C5aR-deficient mice into them. All mice that received wild-type marrow (six of six) but only one of eight mice that received C5aR-deficient marrow developed NCGN (P < 0.05). Albuminuria and neutrophil influx into glomeruli were also significantly attenuated in the mice that received C5aR-deficient marrow (P < 0.05). In summary, C5a and the neutrophil C5aR may compose an amplification loop for ANCA-mediated neutrophil activation. The C5aR may provide a new therapeutic target for ANCA-induced necrotizing crescentic glomerulonephritis.
Figures
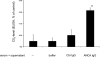

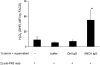

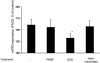
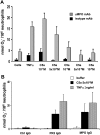
 , TNF-α–primed cells (data from three independent experiments each using two different PR3-ANCA IgG, two different MPO-ANCA IgG, and two different normal IgG, done in duplicate).
, TNF-α–primed cells (data from three independent experiments each using two different PR3-ANCA IgG, two different MPO-ANCA IgG, and two different normal IgG, done in duplicate).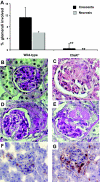
References
-
- Franssen CF, Stegeman CA, Kallenberg CG, Gans RO, De Jong PE, Hoorntje SJ, Tervaert JW: Antiproteinase 3- and antimyeloperoxidase-associated vasculitis. Kidney Int 57: 2195–2206, 2000 - PubMed
-
- Ewert BH, Becker ME, Jennette JC, Falk RJ: Antimyeloperoxidase antibodies induce neutrophil adherence to cultured human endothelial cells. Ren Fail 17: 125–133, 1995 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

