Epoxyeicosatrienoic acid activates BK channels in the cortical collecting duct
- PMID: 19073823
- PMCID: PMC2653685
- DOI: 10.1681/ASN.2008040427
Epoxyeicosatrienoic acid activates BK channels in the cortical collecting duct
Abstract
The cortical collecting duct (CCD), which is involved in renal potassium (K) excretion, expresses cytochrome P450 (CYP)-epoxygenase. Here, we examined the effect of high dietary K on renal expression of CYP2C23 and CYP2J2 in the rat, as well as the role of CYP-epoxygenase-dependent metabolism of arachidonic acid in the regulation of Ca(2+)-activated big-conductance K (BK) channels. By Western blot analysis, high dietary K stimulated the expression of CYP2C23 but not CYP2J2 and increased 11,12-epoxyeicosatrienoic acid (11,12-EET) levels in isolated rat CCD tubules. Application of arachidonic acid increased BK channel activity, and this occurred to a greater extent in rats on a high-K diet compared with a normal-K diet. This effect was unlikely due to arachidonic acid-induced changes in membrane fluidity, because 11,14,17-eicosatrienoic acid did not alter BK channel activity. Inhibiting CYP-epoxygenase but not cyclooxygenase- or CYP-omega-hydroxylase-dependent pathways completely abolished the stimulatory effect of arachidonic acid on BK channel activity. In addition, application of 11,12-EET mimicked the effect of arachidonic acid on BK channel activity, even in the presence of CYP-epoxygenase inhibition. This effect seemed specific to 11,12-EET, because both 8,9- and 14,15-EET failed to stimulate BK channels. Finally, inhibition of CYP-epoxygenase abolished iberiotoxin-sensitive and flow-stimulated but not basal net K secretion in isolated microperfused CCD. In conclusion, high dietary K stimulates the renal CYP-epoxygenase pathway, which plays an important role in activating BK channels and flow-stimulated K secretion in the CCD.
Figures


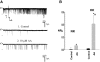
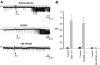




Similar articles
-
Arachidonic acid inhibits basolateral K channels in the cortical collecting duct via cytochrome P-450 epoxygenase-dependent metabolic pathways.Am J Physiol Renal Physiol. 2008 Jun;294(6):F1441-7. doi: 10.1152/ajprenal.00038.2008. Epub 2008 Apr 16. Am J Physiol Renal Physiol. 2008. PMID: 18417544 Free PMC article.
-
Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways.J Gen Physiol. 2004 Dec;124(6):719-27. doi: 10.1085/jgp.200409140. Epub 2004 Nov 15. J Gen Physiol. 2004. PMID: 15545402 Free PMC article.
-
High potassium intake enhances the inhibitory effect of 11,12-EET on ENaC.J Am Soc Nephrol. 2010 Oct;21(10):1667-77. doi: 10.1681/ASN.2009111110. Epub 2010 Jul 1. J Am Soc Nephrol. 2010. PMID: 20595684 Free PMC article.
-
Role of cytochrome P450 epoxygenase in regulating renal membrane transport and hypertension.Curr Opin Nephrol Hypertens. 2013 Mar;22(2):163-9. doi: 10.1097/MNH.0b013e32835d911e. Curr Opin Nephrol Hypertens. 2013. PMID: 23302865 Free PMC article. Review.
-
Epoxyeicosatrienoic acids, 20-hydroxyeicosatetraenoic acid, and renal microvascular function.Prostaglandins Other Lipid Mediat. 2013 Jul-Aug;104-105:2-7. doi: 10.1016/j.prostaglandins.2013.01.002. Epub 2013 Jan 17. Prostaglandins Other Lipid Mediat. 2013. PMID: 23333581 Free PMC article. Review.
Cited by
-
Regulation and function of potassium channels in aldosterone-sensitive distal nephron.Curr Opin Nephrol Hypertens. 2010 Sep;19(5):463-70. doi: 10.1097/MNH.0b013e32833c34ec. Curr Opin Nephrol Hypertens. 2010. PMID: 20601877 Free PMC article. Review.
-
Cyp2c44 epoxygenase is essential for preventing the renal sodium absorption during increasing dietary potassium intake.Hypertension. 2012 Feb;59(2):339-47. doi: 10.1161/HYPERTENSIONAHA.111.178475. Epub 2011 Dec 19. Hypertension. 2012. PMID: 22184322 Free PMC article.
-
Effects of cytochrome P-450 metabolites of arachidonic acid on the epithelial sodium channel (ENaC).Am J Physiol Renal Physiol. 2011 Sep;301(3):F672-81. doi: 10.1152/ajprenal.00597.2010. Epub 2011 Jun 22. Am J Physiol Renal Physiol. 2011. PMID: 21697242 Free PMC article.
-
An unexpected journey: conceptual evolution of mechanoregulated potassium transport in the distal nephron.Am J Physiol Cell Physiol. 2016 Feb 15;310(4):C243-59. doi: 10.1152/ajpcell.00328.2015. Epub 2015 Dec 2. Am J Physiol Cell Physiol. 2016. PMID: 26632600 Free PMC article. Review.
-
Flow-induced prostaglandin E2 release regulates Na and K transport in the collecting duct.Am J Physiol Renal Physiol. 2012 Sep;303(5):F632-8. doi: 10.1152/ajprenal.00169.2012. Epub 2012 Jun 13. Am J Physiol Renal Physiol. 2012. PMID: 22696602 Free PMC article.
References
-
- Giebisch G: Renal potassium transport: Mechanisms and regulation. Am J Physiol Renal Physiol 274: F817–F833, 1998 - PubMed
-
- Palmer LG: Potassium secretion and the regulation of distal nephron K channels. Am J Physiol 277: F821–F825, 1999 - PubMed
-
- Frindt G, Palmer LG: Apical potassium channels in the rat connecting tubule. Am J Physiol Renal Physiol 287: F1030–F1037, 2004 - PubMed
-
- Ho K: The ROM: K-cystic fibrosis transmembrane conductance regulator connection: new insights into the relationship between ROMK and cystic fibrosis transmembrane conductance regulator channels. Curr Opin Nephrol Hypertens 7: 49–58, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

