EGF increases TRPM6 activity and surface expression
- PMID: 19073827
- PMCID: PMC2615736
- DOI: 10.1681/ASN.2008030327
EGF increases TRPM6 activity and surface expression
Abstract
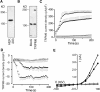
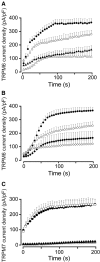
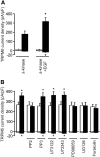
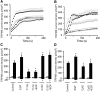
Recent identification of a mutation in the EGF gene that causes isolated recessive hypomagnesemia led to the finding that EGF increases the activity of the epithelial magnesium (Mg2+) channel transient receptor potential M6 (TRPM6). To investigate the molecular mechanism mediating this effect, we performed whole-cell patch-clamp recordings of TRPM6 expressed in human embryonic kidney 293 (HEK293) cells. Stimulation of the EGF receptor increased current through TRPM6 but not TRPM7. The carboxy-terminal alpha-kinase domain of TRPM6 did not participate in the EGF receptor-mediated increase in channel activity. This activation relied on both the Src family of tyrosine kinases and the downstream effector Rac1. Activation of Rac1 increased the mobility of TRPM6, assessed by fluorescence recovery after photobleaching, and a constitutively active mutant of Rac1 mimicked the stimulatory effect of EGF on TRPM6 mobility and activity. Ultimately, TRPM6 activation resulted from increased cell surface abundance. In contrast, dominant negative Rac1 decreased TRPM6 mobility, abrogated current development, and prevented the EGF-mediated increase in channel activity. In summary, EGF-mediated stimulation of TRPM6 occurs via signaling through Src kinases and Rac1, thereby redistributing endomembrane TRPM6 to the plasma membrane. These results describe a regulatory mechanism for transepithelial Mg2+ transport and consequently whole-body Mg2+ homeostasis.
Figures

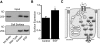
References
-
- Yarden Y, Shilo BZ: SnapShot: EGFR signaling pathway. Cell 131: 1018, 2007 - PubMed
-
- Tejpar S, Piessevaux H, Claes K, Piront P, Hoenderop JG, Verslype C, Van Cutsem E: Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: A prospective study. Lancet Oncol 8: 387–394, 2007 - PubMed
-
- Schrag D, Chung KY, Flombaum C, Saltz L: Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst 97: 1221–1224, 2005 - PubMed
-
- Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG: TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 279: 19–25, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

