Regulation of renal epithelial tight junctions by the von Hippel-Lindau tumor suppressor gene involves occludin and claudin 1 and is independent of E-cadherin
- PMID: 19073886
- PMCID: PMC2633394
- DOI: 10.1091/mbc.e08-06-0566
Regulation of renal epithelial tight junctions by the von Hippel-Lindau tumor suppressor gene involves occludin and claudin 1 and is independent of E-cadherin
Abstract
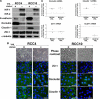
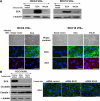
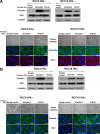
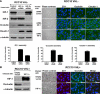
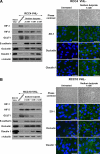
Epithelial-to-mesenchymal transitions (EMT) are important in renal development, fibrosis, and cancer. Loss of function of the tumor suppressor VHL leads to many features of EMT, and it has been hypothesized that the pivotal mediator is down-regulation of the adherens junction (AJ) protein E-cadherin. Here we show that VHL loss-of-function also has striking effects on the expression of the tight junction (TJ) components occludin and claudin 1 in vitro in VHL-defective clear cell renal cell carcinoma (CCRCC) cells and in vivo in VHL-defective sporadic CCRCCs (compared with normal kidney). Occludin is also down-regulated in premalignant foci in kidneys from patients with germline VHL mutations, consistent with a contribution to CCRCC initiation. Reexpression of E-cadherin was sufficient to restore AJ but not TJ assembly, indicating that the TJ defect is independent of E-cadherin down-regulation. Additional experiments show that activation of hypoxia inducible factor (HIF) contributes to both TJ and AJ abnormalities, thus the VHL/HIF pathway contributes to multiple aspects of the EMT phenotype that are not interdependent. Despite the independent nature of the defects, we show that treatment with the histone deacetylase inhibitor sodium butyrate, which suppresses HIF activation, provides a method for reversing EMT in the context of VHL inactivation.
Figures


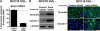
References
-
- Balkovetz D. F. Claudins at the gate: determinants of renal epithelial tight junction paracellular permeability. Am. J. Physiol. Renal Physiol. 2006;290:F572–F579. - PubMed
-
- Bi G., Jiang G. The molecular mechanism of HDAC inhibitors in anticancer effects. Cell Mol. Immunol. 2006;3:285–290. - PubMed
-
- Bilton R., Trottier E., Pouyssegur J., Brahimi-Horn M. C. ARDent about acetylation and deacetylation in hypoxia signalling. Trends Cell Biol. 2006;16:616–621. - PubMed
-
- Braga V. M. Cell-cell adhesion and signalling. Curr. Opin. Cell Biol. 2002;14:546–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

