Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma
- PMID: 19073917
- PMCID: PMC2600580
- DOI: 10.1073/pnas.0810641105
Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma
Abstract
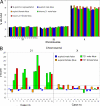
Chromosomal aneuploidy is the major reason why couples opt for prenatal diagnosis. Current methods for definitive diagnosis rely on invasive procedures, such as chorionic villus sampling and amniocentesis, and are associated with a risk of fetal miscarriage. Fetal DNA has been found in maternal plasma but exists as a minor fraction among a high background of maternal DNA. Hence, quantitative perturbations caused by an aneuploid chromosome in the fetal genome to the overall representation of sequences from that chromosome in maternal plasma would be small. Even with highly precise single molecule counting methods such as digital PCR, a large number of DNA molecules and hence maternal plasma volume would need to be analyzed to achieve the necessary analytical precision. Here we reasoned that instead of using approaches that target specific gene loci, the use of a locus-independent method would greatly increase the number of target molecules from the aneuploid chromosome that could be analyzed within the same fixed volume of plasma. Hence, we used massively parallel genomic sequencing to quantify maternal plasma DNA sequences for the noninvasive prenatal detection of fetal trisomy 21. Twenty-eight first and second trimester maternal plasma samples were tested. All 14 trisomy 21 fetuses and 14 euploid fetuses were correctly identified. Massively parallel plasma DNA sequencing represents a new approach that is potentially applicable to all pregnancies for the noninvasive prenatal diagnosis of fetal chromosomal aneuploidies.
Conflict of interest statement
Conflict of interest statement: R.W.K.C., K.C.A.C., N.B.Y.T., F.M.F.L., B.C.Y.Z., C.R.C., and Y.M.D.L. have filed patent applications on the detection of fetal nucleic acids in maternal plasma for noninvasive prenatal diagnosis. Part of this patent portfolio has been licensed to Sequenom. C.R.C. is Chief Scientific Officer of and holds equities in Sequenom. Y.M.D.L is a consultant to and holds equities in Sequenom.
Figures

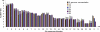
References
-
- Tabor A, et al. Randomised controlled trial of genetic amniocentesis in 4606 low-risk women. Lancet. 1986;1:1287–1293. - PubMed
-
- Malone FD, et al. First-trimester or second-trimester screening, or both, for Down's syndrome. N Engl J Med. 2005;353:2001–2011. - PubMed
-
- Cheung MC, Goldberg JD, Kan YW. Prenatal diagnosis of sickle cell anaemia and thalassaemia by analysis of fetal cells in maternal blood. Nat Genet. 1996;14:264–268. - PubMed
-
- Bianchi DW, et al. Fetal gender and aneuploidy detection using fetal cells in maternal blood: analysis of NIFTY I data. National Institute of Child Health and Development Fetal Cell Isolation Study. Prenat Diagn. 2002;22:609–615. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

