Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats
- PMID: 19074010
- PMCID: PMC2659623
- DOI: 10.1523/JNEUROSCI.2918-08.2008
Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats
Abstract
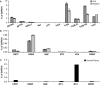
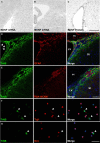
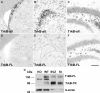
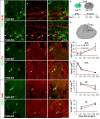
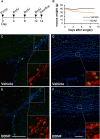
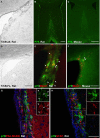
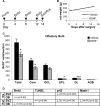
In rodents, the adult subventricular zone (SVZ) generates neuroblasts which migrate to the olfactory bulb (OB) and differentiate into interneurons. Recent work suggests that the neurotrophin Brain-Derived Neurotrophic Factor (BDNF) can enhance adult SVZ neurogenesis, but the mechanism by which it acts is unknown. Here, we analyzed the role of BDNF and its receptor TrkB in adult SVZ neurogenesis. We found that TrkB is the most prominent neurotrophin receptor in the mouse SVZ, but only the truncated, kinase-negative isoform (TrkB-TR) was detected. TrkB-TR is expressed in SVZ astrocytes and ependymal cells, but not in neuroblasts. TrkB mutants have reduced SVZ proliferation and survival and fewer new OB neurons. To test whether this effect is cell-autonomous, we grafted SVZ cells from TrkB knock-out mice (TrkB-KO) into the SVZ of wild-type mice (WT). Grafted progenitors generated neuroblasts that migrated to the OB in the absence of TrkB. The survival and differentiation of granular interneurons and Calbindin(+) periglomerular interneurons seemed unaffected by the loss of TrkB, whereas dopaminergic periglomerular neurons were reduced. Intra-ventricular infusion of BDNF yielded different results depending on the animal species, having no effect on neuron production from mouse SVZ, while decreasing it in rats. Interestingly, mice and rats also differ in their expression of the neurotrophin receptor p75. Our results indicate that TrkB is not essential for adult SVZ neurogenesis and do not support the current view that delivering BDNF to the SVZ can enhance adult neurogenesis.
Figures

References
-
- Anderson KD, Alderson RF, Altar CA, DiStefano PS, Corcoran TL, Lindsay RM, Wiegand SJ. Differential distribution of exogenous BDNF, NGF, and NT-3 in the brain corresponds to the relative abundance and distribution of high-affinity and low-affinity neurotrophin receptors. J Comp Neurol. 1995;357:296–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
