RIM1alpha and RIM1beta are synthesized from distinct promoters of the RIM1 gene to mediate differential but overlapping synaptic functions
- PMID: 19074017
- PMCID: PMC2701653
- DOI: 10.1523/JNEUROSCI.3235-08.2008
RIM1alpha and RIM1beta are synthesized from distinct promoters of the RIM1 gene to mediate differential but overlapping synaptic functions
Abstract
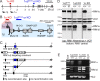
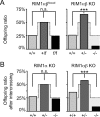
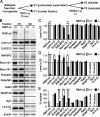
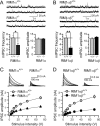
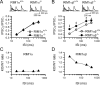
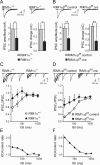
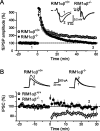
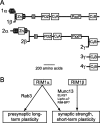
At a synapse, presynaptic terminals form a specialized area of the plasma membrane called the active zone that mediates neurotransmitter release. RIM1alpha is a multidomain protein that constitutes a central component of the active zone by binding to other active zone proteins such as Munc13 s, alpha-liprins, and ELKS, and to synaptic vesicle proteins such as Rab3 and synaptotagmin-1. In mice, knockout of RIM1alpha significantly impairs synaptic vesicle priming and presynaptic long-term plasticity, but is not lethal. We now find that the RIM1 gene encodes a second, previously unknown RIM1 isoform called RIM1beta that is upregulated in RIM1alpha knock-out mice. RIM1beta is identical to RIM1alpha except for the N terminus where RIM1beta lacks the N-terminal Rab3-binding sequence of RIM1alpha. Using newly generated knock-out mice lacking both RIM1alpha and RIM1beta, we demonstrate that different from the deletion of only RIM1alpha, deletion of both RIM1alpha and RIM1beta severely impairs mouse survival. Electrophysiological analyses show that the RIM1alphabeta deletion abolishes long-term presynaptic plasticity, as does RIM1alpha deletion alone. In contrast, the impairment in synaptic strength and short-term synaptic plasticity that is caused by the RIM1alpha deletion is aggravated by the deletion of both RIM1alpha and RIM1beta. Thus, our data indicate that the RIM1 gene encodes two different isoforms that perform overlapping but distinct functions in neurotransmitter release.
Figures


References
-
- Andrews-Zwilling YS, Kawabe H, Reim K, Varoqueaux F, Brose N. Binding to Rab3A-interacting molecule RIM regulates the presynaptic recruitment of Munc13-1 and ubMunc13–2. J Biol Chem. 2006;281:19720–19731. - PubMed
-
- Betz A, Thakur P, Junge HJ, Ashery U, Rhee JS, Scheuss V, Rosenmund C, Rettig J, Brose N. Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron. 2001;30:183–196. - PubMed
-
- Calakos N, Schoch S, Südhof TC, Malenka RC. Multiple roles for the active zone protein RIM1alpha in late stages of neurotransmitter release. Neuron. 2004;42:889–896. - PubMed
-
- Castillo PE, Janz R, Südhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388:590–593. - PubMed
-
- Castillo PE, Schoch S, Schmitz F, Südhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
