Hippocampal network dynamics constrain the time lag between pyramidal cells across modified environments
- PMID: 19074018
- PMCID: PMC2656938
- DOI: 10.1523/JNEUROSCI.3824-08.2008
Hippocampal network dynamics constrain the time lag between pyramidal cells across modified environments
Abstract
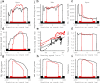
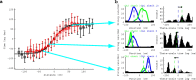
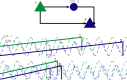
The hippocampus provides a spatial map of the environment. Changes in the environment alter the firing patterns of hippocampal neurons, but are presumably constrained by elements of the network dynamics. We compared the neural activity in CA1 and CA3 regions of the hippocampus in rats running for water reward on a linear track, before and after the track length was shortened. A fraction of cells lost their place fields and new sets of cells with fields emerged, indicating distinct representation of the two tracks. Cells active in both environments shifted their place fields in a location-dependent manner, most notably at the beginning and the end of the track. Furthermore, peak firing rates and place-field sizes decreased, whereas place-field overlap and coactivity increased. Power in the theta-frequency band of the local field potentials also decreased in both CA1 and CA3, along with the coherence between the two structures. In contrast, the theta-scale (0-150 ms) time lags between cell pairs, representing distances on the tracks, were conserved, and the activity of the inhibitory neuron population was maintained across environments. We interpret these observations as reflecting the freedoms and constraints of the hippocampal network dynamics. The freedoms permit the necessary flexibility for the network to distinctly represent unique patterns, whereas the dynamics constrain the speed at which activity propagates between the cell assemblies representing the patterns.
Figures




References
-
- Amari S. Dynamics of pattern formation in lateral-inhibition type neural fields. Biol Cybern. 1977;27:77–87. - PubMed
-
- Bragin A, Csicsvári J, Penttonen M, Buzsáki G. Epileptic afterdischarge in the hippocampal-entorhinal system: current source density and unit studies. Neuroscience. 1997;76:1187–1203. - PubMed
-
- Buzsáki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
