Potential network mechanisms mediating electroencephalographic beta rhythm changes during propofol-induced paradoxical excitation
- PMID: 19074022
- PMCID: PMC2717965
- DOI: 10.1523/JNEUROSCI.3536-08.2008
Potential network mechanisms mediating electroencephalographic beta rhythm changes during propofol-induced paradoxical excitation
Abstract
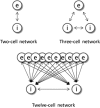
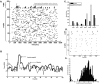
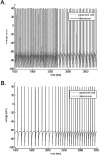
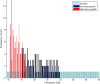
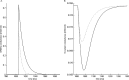
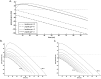
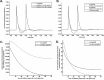
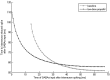
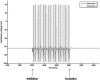
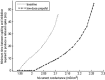
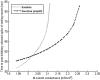
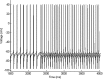
Propofol, like most general anesthetic drugs, can induce both behavioral and electroencephalographic (EEG) manifestations of excitation, rather than sedation, at low doses. Neuronal excitation is unexpected in the presence of this GABA(A)-potentiating drug. We construct a series of network models to understand this paradox. Individual neurons have ion channel conductances with Hodgkin-Huxley-type formulations. Propofol increases the maximal conductance and time constant of decay of the synaptic GABA(A) current. Networks range in size from 2 to 230 neurons. Population output is measured as a function of pyramidal cell activity, with the electroencephalogram approximated by the sum of population AMPA activity between pyramidal cells. These model networks suggest propofol-induced paradoxical excitation may result from a membrane level interaction between the GABA(A) current and an intrinsic membrane slow potassium current (M-current). This membrane level interaction has consequences at the level of multicellular networks enabling a switch from baseline interneuron synchrony to propofol-induced interneuron antisynchrony. Large network models reproduce the clinical EEG changes characteristic of propofol-induced paradoxical excitation. The EEG changes coincide with the emergence of antisynchronous interneuron clusters in the model networks. Our findings suggest interneuron antisynchrony as a potential network mechanism underlying the generation of propofol-induced paradoxical excitation. As correlates of behavioral phenomenology, these networks may refine our understanding of the specific behavioral states associated with general anesthesia.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
