SynGAP regulates steady-state and activity-dependent phosphorylation of cofilin
- PMID: 19074040
- PMCID: PMC2615239
- DOI: 10.1523/JNEUROSCI.4695-08.2008
SynGAP regulates steady-state and activity-dependent phosphorylation of cofilin
Abstract
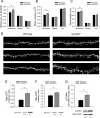
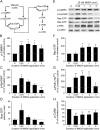
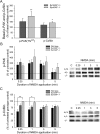
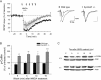
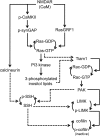
SynGAP, a prominent Ras/Rap GTPase-activating protein in the postsynaptic density, regulates the timing of spine formation and trafficking of glutamate receptors in cultured neurons. However, the molecular mechanisms by which it does this are unknown. Here, we show that synGAP is a key regulator of spine morphology in adult mice. Heterozygous deletion of synGAP was sufficient to cause an excess of mushroom spines in adult brains, indicating that synGAP is involved in steady-state regulation of actin in mature spines. Both Ras- and Rac-GTP levels were elevated in forebrains from adult synGAP(+/-) mice. Rac is a well known regulator of actin polymerization and spine morphology. The steady-state level of phosphorylation of cofilin was also elevated in synGAP(+/-) mice. Cofilin, an F-actin severing protein that is inactivated by phosphorylation, is a downstream target of a pathway regulated by Rac. We show that transient regulation of cofilin by treatment with NMDA is also disrupted in synGAP mutant neurons. Treatment of wild-type neurons with 25 mum NMDA triggered transient dephosphorylation and activation of cofilin within 15 s. In contrast, neurons cultured from mice with a homozygous or heterozygous deletion of synGAP lacked the transient regulation by the NMDA receptor. Depression of EPSPs induced by a similar treatment of hippocampal slices with NMDA was disrupted in slices from synGAP(+/-) mice. Our data show that synGAP mediates a rate-limiting step in steady-state regulation of spine morphology and in transient NMDA-receptor-dependent regulation of the spine cytoskeleton.
Figures

References
-
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. - PubMed
-
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. - PubMed
-
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. - PubMed
-
- Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–187. - PubMed
-
- Chen HJ, Rojas-Soto M, Oguni A, Kennedy MB. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron. 1998;20:895–904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
