Neural substrates of sound-touch synesthesia after a thalamic lesion
- PMID: 19074042
- PMCID: PMC6671766
- DOI: 10.1523/JNEUROSCI.3872-08.2008
Neural substrates of sound-touch synesthesia after a thalamic lesion
Abstract
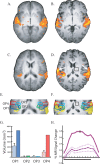
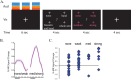
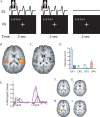
Neural plasticity induced by stroke can mediate positive outcomes, such as recovery of function, but can also result in the formation of abnormal connections with negative consequences for perception and cognition. In three experiments using blood-oxygen level dependent (BOLD) functional magnetic resonance imaging, we examined the neural substrates of acquired auditory-tactile synesthesia, in which certain sounds can produce an intense somatosensory tingling sensation in a patient with a thalamic lesion. Compared with nine normal controls, the first experiment showed that the patient had a threefold greater BOLD response to sounds in the parietal operculum, the location of secondary somatosensory cortex. We hypothesized that this abnormal opercular activity might be the neural substrate of the patient's synesthesia. Supporting this hypothesis, the second experiment demonstrated that sounds that produced no somatosensation did not evoke a BOLD response in the operculum, while sounds that produced strong somatosensations evoked large BOLD responses. These abnormal responses may have resulted from plasticity induced by the loss of somatosensory inputs. Consistent with this idea, in the third experiment, BOLD responses to somatosensory stimulation were significantly weaker in the patient's operculum than in normal controls. These experiments demonstrate a double dissociation in the patient's secondary somatosensory cortex (increased responses to auditory stimulation and decreased responses to somatosensory stimulation), and suggest both that stroke-induced plasticity can result in abnormal connections between sensory modalities that are normally separate, and that synesthesia can be caused by inappropriate connections between nearby cortical territories.
Figures
References
-
- Aglioti S, Cortese F, Franchini C. Rapid sensory remapping in the adult human brain as inferred from phantom breast perception. Neuroreport. 1994;5:473–476. - PubMed
-
- Armel KC, Ramachandran R. Acquired synesthesia in retinitis pigmentosa. Neurocase. 1999;5:293–296.
-
- Beauchamp MS, Lee KE, Argall BD, Martin A. Integration of auditory and visual information about objects in superior temporal sulcus. Neuron. 2004a;41:809–823. - PubMed
-
- Beauchamp MS, Argall BD, Bodurka J, Duyn JH, Martin A. Unraveling multisensory integration: patchy organization within human STS multisensory cortex. Nat Neurosci. 2004b;7:1190–1192. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
