Solution NMR structure of the C-terminal EF-hand domain of human cardiac sodium channel NaV1.5
- PMID: 19074138
- PMCID: PMC2649110
- DOI: 10.1074/jbc.M807747200
Solution NMR structure of the C-terminal EF-hand domain of human cardiac sodium channel NaV1.5
Abstract
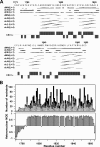
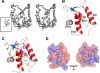
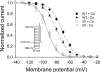
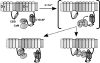
The voltage-gated sodium channel NaV1.5 is responsible for the initial upstroke of the action potential in cardiac tissue. Levels of intracellular calcium modulate inactivation gating of NaV1.5, in part through a C-terminal EF-hand calcium binding domain. The significance of this structure is underscored by the fact that mutations within this domain are associated with specific cardiac arrhythmia syndromes. In an effort to elucidate the molecular basis for calcium regulation of channel function, we have determined the solution structure of the C-terminal EF-hand domain using multidimensional heteronuclear NMR. The structure confirms the existence of the four-helix bundle common to EF-hand domain proteins. However, the location of this domain is shifted with respect to that predicted on the basis of a consensus 12-residue EF-hand calcium binding loop in the sequence. This finding is consistent with the weak calcium affinity reported for the isolated EF-hand domain; high affinity binding is observed only in a construct with an additional 60 residues C-terminal to the EF-hand domain, including the IQ motif that is central to the calcium regulatory apparatus. The binding of an IQ motif peptide to the EF-hand domain was characterized by isothermal titration calorimetry and nuclear magnetic resonance spectroscopy. The peptide binds between helices I and IV in the EF-hand domain, similar to the binding of target peptides to other EF-hand calcium-binding proteins. These results suggest a molecular basis for the coupling of the intrinsic (EF-hand domain) and extrinsic (calmodulin) components of the calcium-sensing apparatus of NaV1.5.
Figures


References
-
- Yu, F. H., and Catterall, W. A. (2003) Genome Biology http://genomebiology.com/2003/4/3/207 - PMC - PubMed
-
- Abriel, H., Cabo, C., Wehrens, X. H., Rivolta, I., Motoike, H. K., Memmi, M., Napolitano, C., Priori, S. G., and Kass, R. S. (2001) Circ. Res. 88 740-745 - PubMed
-
- Wehrens, X. H., Abriel, H., Cabo, C., Benhorin, J., and Kass, R. S. (2000) Circulation 102 584-590 - PubMed
-
- Priori, S. G., Napolitano, C., Gasparini, M., Pappone, C., Della Bella, P., Giordano, U., Bloise, R., Giustetto, C., De Nardis, R., Grillo, M., Ronchetti, E., Faggiano, G., and Nastoli, J. (2002) Circulation 105 1342-1347 - PubMed
-
- Vatta, M., Dumaine, R., Antzelevitch, C., Brugada, R., Li, H., Bowles, N. E., Nademanee, K., Brugada, J., Brugada, P., and Towbin, J. A. (2002) Mol. Genet. Metab. 75 317-324 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

